Abstract
Purpose
The research aimed to determine the transport velocities of aniline (AN) and nitrobenzene (NB) in sandy sediment so as to predict the pollution plume in sandy aquifer. The transport velocities of organic compounds in aquifer depend on various factors. This research also evaluated the effects of compound type, single or mixture solute systems, pore-water velocity (v), and pH on the transport velocities of AN and NB. These results will facilitate more precise determination of transport velocities.
Materials and methods
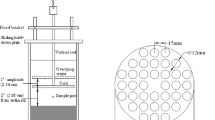
Column experiments containing sorption and desorption periods were conducted using typical sandy sediment from JiangHan Plain, China. Special emphasis was placed on the variation of compounds, solute systems, v from 4.5 to 10 cm h−1, and pH from 5 to 8.5. The transport curves of AN and NB were simulated using different sorption models coupled in HYDRUS-1D and the partition coefficient, Kd, was determined by inverse solution. The retardation coefficient (R) and transport velocity (1/R) were calculated based on Kd and the basic properties of the column. The transport velocities of AN and NB in various experimental conditions were compared and discussed.
Results and discussion
The linear non-equilibrium two-site (L2Site) sorption model coupled in HYDRUS-1D best described the sorption of AN and NB during the transport process in the sandy sediment. The 1/R values of AN and NB were 0.47–0.67 m and 0.16–0.21 m, respectively. AN transported three times faster than NB in both single and mixture systems. Lower v enhanced the transport of AN but displayed limited impact on the transport of NB in single solute system. In mixture solute system, the 1/R values of AN tended to decrease and NB increase at lower v, which suggested an interference between AN and NB transports. Both transport velocities of AN and NB potentially increase with pH.
Conclusions
AN and NB can transport 0.47–0.67 m and 0.16–0.21 m, respectively, when groundwater flows 1 m at various experimental conditions. The transport velocities of AN and NB depend on the type of compounds, single or mixture solute system, v, and pH. All the factors should be considered when we determine the parameter, transport velocities, for prediction of pollution plume.




Similar content being viewed by others
References
An FQ, Feng XQ, Gao BJ (2009) Adsorption of aniline from aqueous solution using novel adsorbent PAM/SiO2. Chem Eng J 151:183–187
ATSDR (1990) Toxicological profile for nitrobenzene. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Burke V, Treumann S, Duennbier U, Greskowiak J, Massmann G (2013) Sorption behavior of 20 wastewater originated micropollutants in groundwater—column experiments with pharmaceutical residues and industrial agents. J Contam Hydrol 154:29–41
Cao XY, Pang HL, Yang GP (2015) Sorption behaviour of norfloxacin on marine sediments. J Soils Sediments 15:1635–1643
Chen M, Cui L, Li C, Diao G (2009) Adsorption, desorption and condensation of nitrobenzene solution from active carbon: a comparison of two cyclodextrins and two surfactants. J Hazard Mater 162:23–28
China News (2013) Local newspaper report, Hebei “Red color groundwater” aniline concentration exceeded more than 70 times the standard. Available at: http://www.chinanewscom/sh/2013/04–08/4710431shtml. Accessed 11 July 2015
Contreras S, Rodriguez M, Chamarro E, Esplugas S (2001) UV- and UV/Fe(III)-enhanced ozonation of nitrobenzene in aqueous solution. J Photochem Photobiol A 142:79–83
Dong J, Dong Y, Wen C, Gao S, Ren L, Bao Q (2018) A 2D tank test on remediation of nitrobenzene-contaminated aquifer using in-situ reactive zone with emulsified nanoscale zero-valent iron. Chemosphere 206:766–776
Florido A, Valderrama C, Arevalo JA, Casas I, Martinez M, Miralles N (2010) Application of two sites non-equilibrium sorption model for the removal of Cu(II) onto grape stalk wastes in a fixed-bed column. Chem Eng J 156:298–304
Gong R, Liang J, Chen J, Huang F (2009) Removal of bisphenol A from aqueous solution by hydrophobic sorption of hemimicelles. Int J Environ Sci Technol 6:539–544
Gurten AA, Ucan S, Ozler MA, Ayar A (2005) Removal of aniline from aqueous solution by PVC-CDAE ligand-exchanger. J Hazard Mater 120:81–87
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR hydrophobic, electronic and steric constants. ACS, Washington, DC
Ko CH, Fan C, Chiang PN, Wang MK, Lin KC (2007) P-Nitrophenol, phenol and aniline sorption by organo-clays. J Hazard Mater 149:275–282
Kumar M, Tamilarasan R (2013) Modeling studies: adsorption of aniline blue by using Prosopis juliflora carbon/Ca/alginate polymer composite beads. Carbohydr Polym 92:2171–2180
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275
Liu H, Zhang D, Li MJ, Tong L, Feng L (2013) Competitive adsorption and transport of phthalate esters in the clay layer of JiangHan Plain, China. Chemosphere 92:1542–1549
Liu H, Li Y, He X, Sissou Z, Tong L, Yarnes C, Huang X (2016) Compound-specific carbon isotopic fractionation during transport of phthalate esters in sandy aquifer. Chemosphere 144:1831–1836
Maraqa MA, Zhao X, Lee J-u, Allan F, Voice TC (2011) Comparison of nonideal sorption formulations in modeling the transport of phthalate esters through packed soil columns. J Contam Hydrol 125:57–69
O’Brien J, O'Dwyer TF, Curtin T (2008) A novel process for the removal of aniline from wastewaters. J Hazard Mater 159:476–482
Oh S, Shin WS, Kim HT (2016) Effects of pH, dissolved organic matter, and salinity on ibuprofen sorption on sediment. Environ Sci Pollut Res 23:22882–22889
Palma G, Demanet R, Jorquera M, Mora ML, Briceno G, Violante A (2015) Effect of pH on sorption kinetic process of acidic herbicides in a volcanic soil. J Soil Sci Plant Nutr 15:549–560
Pan JJ, Guan BH (2010) Adsorption of nitrobenzene from aqueous solution on activated sludge modified by cetyltrimethylammonium bromide. J Hazard Mater 183:341–346
Podkoscielny R, Laszlo K (2007) Heterogeneity of activated carbons in adsorption of aniline from aqueous solutions. Appl Surf Sci 253:8762–8771
Qin Q, Ma J, Liu K (2007) Adsorption of nitrobenzene from aqueous solution by MCM-41. J Colloid Interface Sci 315:80–86
Qu Y, Yang H, Wang S, Chen T, Wang G (2017) Hydrogenation of nitrobenzene to aniline catalyzed by C60-stabilized Ni. Catal Commun 97:83–87
Rauthula MS, Srivastava VC (2011) Studies on adsorption/desorption of nitrobenzene and humic acid onto/from activated carbon. Chem Eng J 168:35–43
Saffron CM, Park J-H, Dale BE, Voice TC (2006) Kinetics of contaminant desorption from soil: comparison of model formulations using the Akaike information criterion. Environ Sci Technol 40:7662–7667
Shepherd BO, Erler DV, Tait DR, van Zwieten L, Kimber S, Eyre BD (2015) Behaviour of estrogenic endocrine-disrupting chemicals in permeable carbonate sands. Environ Sci Pollut Res 22:11340–11348
Simunek J, van Genuchten MT, Sejna M (2005) The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media. University of California, Riverside, Riverside
Styszko K (2016) Sorption of emerging organic micropollutants onto fine sediments in a water supply dam reservoir, Poland. J Soils Sediments 16:677–686
Wang S, Yang S, Jin X, Liu L, Wu F (2010) Use of low cost crop biological wastes for the removal of nitrobenzene from water. Desalination 264:32–36
Wang P, Hua Z, Cai Y, Shen X, Li Q, Liu X (2015) Effects of hydrodynamic conditions on the sorption behaviors of aniline on sediment with coexistence of nitrobenzene. Environ Sci Pollut Res 22:11595–11605
Wang K, Larkin T, Singhal N, Song Y (2018) Mobility of pharmaceutical and personal care products in lime amended wastewater biosolids. Sci Total Environ 624:1263–1273
Wei W, Sun R, Cui J, Wei ZG (2010) Removal of nitrobenzene from aqueous solution by adsorption on nanocrystalline hydroxyapatite. Desalination 263:89–96
Yang H, Hu Y, Cheng H (2016) Sorption of chlorophenols on microporous minerals: mechanism and influence of metal cations, solution pH, and humic acid. Environ Sci Pollut Res 23:19266–19280
Zakari S, Liu H, Li YX, He X, Tong L (2016a) Transport and sorption behavior of individual phthalate esters in sandy aquifer: column experiments. Environ Sci Pollut Res 23:15749–15756
Zakari S, Liu H, Tong L, Wang Y, Liu J (2016c) Transport of bisphenol-A in sandy aquifer sediment: column experiment. Chemosphere 144:1807–1814
Zheng H, Liu D, Zheng Y, Liang S, Liu Z (2009) Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J Hazard Mater 167:141–147
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant nos. 41830862, 41521001), Hubei Province for Innovative Research Groups (grant no. 2018CFA028), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (CUG170103).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jan Schwarzbauer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 340 kb)
Rights and permissions
About this article
Cite this article
Zakari, S., Liu, H. & Zhou, H. Transport velocities of aniline and nitrobenzene in sandy sediment. J Soils Sediments 19, 2570–2579 (2019). https://doi.org/10.1007/s11368-019-02287-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02287-6