Abstract
Purpose
Several interactions between Al and the solid phase of soil influence Al buffering in soil solution. This work evaluated soils cultivated with Pinus taeda L. to determine Al forms in organic and mineral horizons using various extraction methods and to relate acidity with clay mineralogy.
Materials and methods
Organic and mineral horizons of 10 soil profiles (up to 2.1 m deep) in southern Brazil were sampled. Organic horizons were separated into fresh, aged, and fermented/humified litter. The following Al extraction methods were utilized: 0.5 mol L−1 pH 2.8 CuCl2–Al complexed in organic matter; 1.0 mol L−1 KCl–exchangeable Al; water–Al soluble in soil solution; HF concentrated + HNO3 concentrated + H2O2 30% (v/v)–total Al. Six sequential extractions were carried out to isolate different forms of amorphous minerals that can buffer Al on soil solution: 0.05 and 0.1 mol L−1 sodium pyrophosphate; 0.1 and 0.2 mol L−1 ammonium oxalate; 0.25 and 0.5 mol L−1 NaOH. Samples of clay were also analyzed by XRD.
Results and discussion
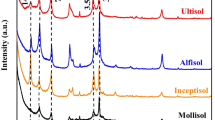
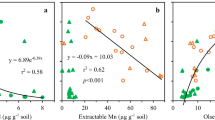
There was a clear effect of litter age on increasing total Al concentration. In the aged litter and fermented and/or humified litter, levels of total Al were 1.4 to 3.8 and 1.5 to 7.8 times greater than in fresh litter, respectively. The CuCl2 method had higher Al extraction capacity than the KCl method for litter. The lowest Al–pyrophosphate values were observed in the Oxisol, which also had a predominance of gibbsite and the lowest levels of Al–KCl and Al–CuCl2. There was an inverse relationship between degree of soil weathering and soluble and exchangeable Al in soils. Available Al increased with higher Si proportion in minerals of the clay fraction (2:1 > 1:1 > 0:1).
Conclusions
The worst scenario was soils with the combination of high soluble and exchangeable Al levels and high concentrations of amorphous forms of Al minerals. The best predictors of Al accumulation in the youngest litter horizon were extractions of amorphous minerals with pyrophosphate and NaOH. These extractors are normally used to predict the level of Al buffering in soils. Organic matter had less influence on Al dynamics in soils.



Similar content being viewed by others
References
Bloom PR, McBride MB, Weaver RM (1979) Aluminum organic matter in acid soils: salt–extractable aluminum. Soil Sci Soc Am J 43:813–815
Brunner I, Sperisen C (2013) Aluminum exclusion and aluminum tolerance in woody plants. Front Plant Sci 4:172–179
Dahlgren RA (1994) Quantification of allophane and imogolite. In: Amonette JE, Zelazny LW (eds) Quantitative methods in soil mineralogy. Soil Science Society of America, Madson, pp 430–450
Eimil-Fraga C, Álvarez-Rodríguez E, Rodríguez-Soalleiro R, Fernández-Sanjurjo MJ (2015) Influence of parent material on the aluminium fractions in acidic soils under Pinus pinaster in Galicia (NW Spain). Geoderma 255:50–57
Embrapa – Empresa Brasileira de Pesquisa Agropecuária (1997) Handbook of analysis of soil methods, 2nd edn. Embrapa/CNPS, Rio de Janeiro, Brazil
Foy CD, Fleming AL (1978) The physiology of plant tolerance to excess available aluminum and manganese in acid soils. In: Jung GA (ed) Crop tolerance to suboptimal land conditions, ASA spec Publ 32. ASA, CSSA, and SSSA, Madison, pp 301–328. https://doi.org/10.2134/asaspecpub32.c14
García-Rodeja E, Nóvoa JC, Pontevedra X, Martínez-Cortizas A, Buurman P (2004) Aluminium fractionation of European volcanic soils by selective dissolution techniques. Catena 56:155–183
Gee GW, Bauder JW (1986) Particle–size analysis. In: Klute A (ed) Methods of soil analysis part 1: physical and mineralogical methods. ASA, Madison, pp 383–411
Goya J, Pérez C, Fernández R (2008) Concentración foliar de nutrientes en plantaciones de diferentes edades de Pinus taeda L. en el norte de Misiones, Argentina. XIII Jornadas Técnicas Forestales y Ambientales, UNAM–INTA. Eldorado, Misiones, Argentina. ISSN,16685385
Hargrove WL, Thomas GW (1984) Extraction of aluminum from aluminum–organic matter in relation to titratable acidity. Soil Sci Soc Am J 48:1458–1460
Jackson ML, Lim CH, Zelazny LW (1986) Oxides, hydroxides, and aluminosilicates. In: Klute A (ed) Methods of soil analysis part 1: physical and mineralogical methods. ASA, Madison, pp 101–150
Jonczak J (2012) Effect of pine admixture in a beech stand on the intensity of dissolved organic carbon, iron and aluminium leaching from organic and humic horizons of dystric Arenosols. For Res Pap 73:143–151
Juo ASR, Kamprath EJ (1979) Copper chloride as an extractant for estimating the potentially reactive aluminum pool in acid soils. Soil Sci Soc Am J 43:35–38
Kirkland DL, Hajek BF (1972) Formula derivation of Al–interlayered vermiculite in selected soil clays. Soil Sci 114:317–322
Lindsay WL (1979) Chemical equilibria in soils. John Wiley and Sons, New York
Martins AP, Reissmann CB (2007) Plant material and laboratory routines in chemical-analytical procedures. Sci Agrár 8:56–61
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a ditionite–citrate system buffered with sodium bicarbonate. Clay Miner 7:317–327
Melo VF, Schaefer CE, Novais RF, Singh B, Fontes MPF (2002) Potassium and magnesium in clay minerals of some Brazilian soils as indicated by a sequential extraction procedure. Commun Soil Sci Plant Anal 33:2203–2225
Mendonça T, Melo VF, Alleoni LR, Schaefer CE, Michel RF (2013) Lead adsorption in the clay fraction of two soil profiles from Fildes peninsula, King George Island. Antarct Sci 25:389–396
Mládková L, Boruvka L, Drábek O (2004) Distribution of aluminium among its mobilizable forms in soils of the Jizera mountains region. Plant Soil Environ 50:346–351
Poggere GC, Melo VF, Francelino MR, Schaefer CE, Simas FN (2016) Characterization of products of the early stages of pedogenesis in ornithogenic soil from maritime Antarctica. Eur J Soil Sci 67:70–78
Poggere GC, Melo VF, Curi N, Schaefer CE, Francelino MR (2017) Adsorption and desorption of lead by low–crystallinity colloids of Antarctic soils. Appl Clay Sci 146:371–379
Poppe LJ, Paskevich VF, Hathaway JC, Blackwood DS (2004) A laboratory manual for X–ray powder diffraction. US Geological Survey Open–File Report. US Department of the Interior, US Geological Survey, Woods Hole
Rabel DO, Motta ACV, Barbosa JZ, Melo VF, Prior ST (2018) Depth distribution of exchangeable aluminum in acid soils: a study from subtropical Brazil. Acta Sci Agron 40:39320. https://doi.org/10.4025/actasciagron.v40i1.39320
Ramos MR, Melo VF, Uhlmann A, Dedecek RA, Curcio GR (2015) Clay mineralogy and genesis of fragipan in soils from Southeast Brazil. Catena 135:22–28
Schwertmann U (1973) Use of oxalate for Fe extraction from soils. Can J Soil Sci 53:244–246
Silva S (2012) Aluminium toxicity targets in plants. J Bot 2012:1–8. https://doi.org/10.1155/2012/219462
Tarì G, Bobos I, Gomes CSF, Ferreira JMF (1999) Modification of surface charge properties during kaolinite to halloysite–7Å transformation. J Colloid Interface Sci 210:360–366
Tejnecký V, Drábek O, Borůvka L, Nikodem A, Kopáč J, Vokurková P, Šebek O (2010) Seasonal variation of water extractable aluminium forms in acidified forest organic soils under different vegetation cover. Biogeochemistry 101:151–163
USEPA – United States Environmental Protection Agency(1997) Method 3052 – Microwave assisted acid digestion of siliceous and organically based matrices. http://www.epa.gov/solidwaste/hazard/testmethods/sw846/pdfs/3052.pdf
Vance GF, Stevensen FJ, Sikora FJ (1996) Environmental chemistry of aluminum–organic complexes. In: Sposito G (ed) The environmental chemistry of aluminum. Lewis Publishers, Boca Raton, pp 169–220
Walker WJ, Cronan CS, Bloom PR (1990) Aluminum solubility in organic soil horizons from northern and southern forested watersheds. Soil Sci Soc Am J 54:369–374
Zołotajkin M, Ciba J, Kluczka J, Skwira M, Smoliński A (2011) Exchangeable and bioavailable aluminium in the mountain forest soil of Barania Góra range (Silesian Beskids, Poland). Water Air Soil Pollut 216:571–580
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yanzheng Gao
Rights and permissions
About this article
Cite this article
Rodrigues, A.N.A., Motta, A.C.V., Melo, V.F. et al. Forms and buffering potential of aluminum in tropical and subtropical acid soils cultivated with Pinus taeda L. J Soils Sediments 19, 1355–1366 (2019). https://doi.org/10.1007/s11368-018-2144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2144-7