Abstract
Purpose
Few studies have described the bacterial community structures of turbid rivers. In this paper, the characteristics of the bacterial community in the water and surface sediment of the Yellow River, China, the largest turbid river in the world, were studied.
Materials and methods
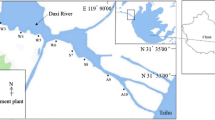
Water and sediment samples were collected from six sites along the river. Bacterial community composition was determined using the 16S ribosomal RNA (rRNA) gene clone library technique. The relationship between environmental parameters and bacterial diversity was analyzed.
Results and discussion
A total of 1,131 gene sequences were obtained and clustered into 639 operational taxonomic units (at the 97 % identity level), with Proteobacteria as the predominant phylum. The Shannon index for water samples ranged from 3.39 to 4.40 and was generally higher than that in other rivers; this was probably due to the high suspended particulate sediment (SPS) concentration in the Yellow River, which can provide more habitats for both aerobic and anaerobic bacteria. Also, the bacterial diversity of the water samples was slightly higher than that of the surface sediment samples. The bacterial diversity of water increased along the river in the downstream direction, while there was no trend for the sediment. Redundancy analysis indicated that pH, dissolved organic carbon (DOC), and SPS were the main factors controlling the water bacterial community in the Yellow River, and pH, nitrate–nitrogen, and water content were the main factors for the surface sediment bacterial community.
Conclusions
This study indicated that the bacterial diversity of the Yellow River is generally higher than that in other rivers, suggesting that SPS plays an important role in regulating bacterial diversity and community structure in aquatic environments.





Similar content being viewed by others
References
Baik KS, Park SC, Kim EM, Bae KS, Ann J-H, Ka J-O, Chun J, Seong CN (2008) Diversity of bacterial community in freshwater of Woopo wetland. J Microbiol 46:647–655
Beier S, Witzel K-P, Marxsen J (2008) Bacterial community composition in central European running waters examined by temperature gradient gel electrophoresis and sequence analysis of 16S rRNA genes. Appl Environ Microb 74:188–199
Benner R, Strom M (1993) A critical evaluation of the analytical blank associated with DOC measurements by high-temperature catalytic oxidation. Mar Chem 41:153–160
Brümmer I, Fehr W, Wagner-Döbler I (2000) Biofilm community structure in polluted rivers: abundance of dominant phylogenetic groups over a complete annual cycle. Appl Environ Microb 66:3078–3082
Chiaramonte JB, do Carmo Roberto M, Pagioro TA (2013) Seasonal dynamics and community structure of bacterioplankton in Upper Paraná River floodplain. Microb Ecol 66:773–783
Cole JK, Peacock JP, Dodsworth JA, Williams AJ, Thompson DB, Dong H, Wu G, Hedlund BP (2013) Sediment microbial communities in Great Boiling Spring are controlled by temperature and distinct from water communities. ISME J 4:718–729
Cottrell MT, Waidner LA, Yu L, Kirchman DL (2005) Bacterial diversity of metagenomic and PCR libraries from the Delaware River. Environ Microbiol 7:1883–1895
Crump BC, Hobbie JE (2005) Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50:1718–1729
Crump BC, Peterson BJ, Raymond PA, Amon RM, Rinehart A, McClelland JW, Holmes RM (2009) Circumpolar synchrony in big river bacterioplankton. Proc Natl Acad Sci 106:21208–21212
Feng BW, Li XR, Wang JH, Hu ZY, Meng H, Xiang LY, Quan ZX (2009) Bacterial diversity of water and sediment in the Changjiang estuary and coastal area of the East China Sea. FEMS Microbiol Ecol 70:236–248
Galand PE, Lovejoy C, Pouliot J, Garneau M-E, Vincent WF (2008) Microbial community diversity and heterotrophic production in a coastal Arctic ecosystem: a stamukhi lake and its source waters. Limnol Oceanogr 53:813
Hewson I, Fuhrman JA (2004) Richness and diversity of bacterioplankton species along an estuarine gradient in Moreton Bay, Australia. Appl Environ Microb 70:3425–3433
Hollister EB, Engledow AS, Hammett AJM, Provin TL, Wilkinson HH, Gentry TJ (2010) Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J 4:829–838
Huang S, Chen C, Wu Y, Wu Q, Zhang R (2011) Characterization of depth-related bacterial communities and their relationships with the environmental factors in the river sediments. J Microbiol Biotechnol 27:2655–2664
Hugenholtz P, Goebel BM, Pace NR (1998) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180:4765–4774
Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl Environ Microb 72:3832–3845
Kirchman DL, Dittel AI, Findlay SE, Fischer D (2004) Changes in bacterial activity and community structure in response to dissolved organic matter in the Hudson River, New York. Aquat Microb Ecol 35:243–257
Kobayashi Y, Kim C, Yoshimizu C, Kohzu A, Tayasu I, Nagata T (2009) Longitudinal changes in bacterial community composition in river epilithic biofilms: influence of nutrients and organic matter. Aquat Microb Ecol 54:135
Köllner KE, Carstens D, Schubert CJ, Zeyer J, Bürgmann H (2013) Impact of particulate organic matter composition and degradation state on the vertical structure of particle-associated and planktonic lacustrine bacteria. Aquat Microb Ecol 69:81–92
Lane D (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Liu SD, Xia XH, Zhai YW, Wang R, Liu T, Zhang SW (2011) Black carbon (BC) in urban and surrounding rural soils of Beijing, China: spatial distribution and relationship with polycyclic aromatic hydrocarbons (PAHs). Chemosphere 82:223–228
Liu T, Xia XH, Liu SD, Mou XL, Qiu YW (2013) Acceleration of denitrification in turbid rivers due to denitrification occurring on suspended sediment in oxic waters. Environ Sci Technol 47:4052–4061
Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci 104:11436–11440
Maidak BL, Cole JR, Lilburn TG, Parker CT Jr, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM (2001) The RDP-II (ribosomal database project). Nucleic Acid Res 29:173–174
Medihala PG, Lawrence JR, Swerhone GDW, Korber DR (2012) Spatial variation in microbial community structure, richness, and diversity in an alluvial aquifer. Can J Microbiol 58:1135–1151
Nam Y-D, Sung Y, Chang H-W, Roh SW, Kim K-H, Rhee S-K, Kim J-C, Kim J-Y, Yoon J-H, Bae J-W (2008) Characterization of the depth-related changes in the microbial communities in Lake Hovsgol sediment by 16S rRNA gene-based approaches. J Microbiol 46:125–136
Nealson KH (1997) Sediment bacteria: who’s there, what are they doing, and what’s new? Annu Rev Earth Plent Sci 25:403–434
Portillo MC, Anderson SP, Fierer N (2012) Temporal variability in the diversity and composition of stream bacterioplankton communities. Environ Microbiol 14:2417–2428
Putnam JE, Pope LM (2003) Trends in suspended-sediment concentration at selected stream sites in Kansas, 1970–2002. US Department of the Interior, US Geological Survey, Denver, CO, Water-Resources Investigations Report 03–4150, 36 pp
Rubin MA, Leff LG (2007) Nutrients and other abiotic factors affecting bacterial communities in an Ohio River (USA). Microb Ecol 54:374–383
Ruiz‐González C, Proia L, Ferrera I, Gasol JM, Sabater S (2013) Effects of large river dam regulation on bacterioplankton community structure. FEMS Microbiol Ecol 84:316–331
Salles JF, Van Veen JA, Van Elsas JD (2004) Multivariate analyses of Burkholderia species in soil: effect of crop and land use history. App Environ Microb 70:4012–4020
Sánchez-Andrea I, Rodríguez N, Amils R, Sanz JL (2011) Microbial diversity in anaerobic sediments at Rio Tinto, a naturally acidic environment with a high heavy metal content. Appl Environ Microbiol 77:6085–6093
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7537–7541
Sekiguchi H, Watanabe M, Nakahara T, Xu B, Uchiyama H (2002) Succession of bacterial community structure along the Changjiang River determined by denaturing gradient gel electrophoresis and clone library analysis. Appl Environ Microbiol 68:5142–5150
Sivakumar B (2002) A phase-space reconstruction approach to prediction of suspended sediment concentration in rivers. J Hydrol 258:149–162
Wang S, Dong RM, Dong CZ, Huang L, Jiang H, Wei Y, Feng L, Liu D, Yang G, Zhang C (2012) Diversity of microbial plankton across the Three Gorges Dam of the Yangtze River, China. Geosci Front 3:335–349
Winter C, Hein T, Kavka G, Mach RL, Farnleitner AH (2007) Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Appl Environ Microb 73:421–431
Wu P, Wang Y-S, Sun F-L, Wu M-L, Y-l P (2013) Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases in the sediments from the Pearl River estuary, China. Appl Microbial Biotechnol. doi:10.1007/s00253-013-4854-5
Xia XH, Zhou J, Yang Z (2002) Nitrogen contamination in the Yellow River basin of China. J Environ Qual 31:917–925
Xia XH, Meng LH, Yang ZF (2005) Influence of humic substance in solids on the measurement of oxygen-consuming organics of the Yellow River. J Environ Inform 6:51–57
Xia XH, Yang ZF, Zhang XQ (2009) Effect of suspended-sediment concentration on nitrification in river water: importance of suspended sediment−water interface. Environ Sci Technol 43:3681–3687
Xia X, Zhou Z, Zhou CH, Jiang G, Liu T (2011) Effects of suspended sediment on the biodegradation and mineralization of phenanthrene in river water. J Environ Qual 40:118–125
Yang S, Li H, Ysebaert T, Bouma T, Zhang W, Wang Y, Li P, Li M, Ding P (2008) Spatial and temporal variations in sediment grain size in tidal wetlands, Yangtze Delta: on the role of physical and biotic controls. Estuar Coast Shelf Sci 77:657–671
Ye W, Liu X, Lin S, Tan J, Pan J, Li D, Yang H (2009) The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiol Ecol 70:263–276
YRCC (Yellow River Conservancy Commission) (2010) Yellow River sediment bulletin. YRCC, Zhengzhou (In Chinese)
Zwart G, Crump BC, Kamst-van Agterveld MP, Hagen F, Han S-K (2002) Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol 28:141–155
Acknowledgements
The study was supported by the Major State Basic Research Development Program (2010CB951104), National Science Foundation for Distinguished Young Scholars (51325902), National Science Foundation for Innovative Research Group (51121003), and National Science Foundation of China (51279010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: John R. Lawrence
Rights and permissions
About this article
Cite this article
Xia, N., Xia, X., Liu, T. et al. Characteristics of bacterial community in the water and surface sediment of the Yellow River, China, the largest turbid river in the world. J Soils Sediments 14, 1894–1904 (2014). https://doi.org/10.1007/s11368-014-0974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0974-5