Abstract
Purpose
Using reactive industrial by-products (IBPs) to reduce phosphorus (P) losses associated with diffuse water pollution is a potentially cost-effective mitigation strategy. However, IBPs must be screened to assess their effectiveness and optimum application rates. This requires accurate estimates of parameters such as the maximum P sorption capacity. Traditionally, these parameters have been derived from the Langmuir model applied to data from batch sorption experiments following a 24-h equilibration period. In this paper, we examined (i) how equilibration time can influence estimates of the maximum P sorption capacity for IBPs and (ii) the relative P sorption characteristics of a range of IBPs available in the UK.
Materials and methods
Four IBPs containing different reactive components including ochre, aluminium (Al)-based water treatment residual (WTR), iron (Fe)-based WTR and Fe-lime (CaO)-based WTR were selected for this study. The maximum P sorption capacities of these IBPs were determined using a linearized Langmuir model applied to batch sorption data collected at different equilibration times of 24 h, 5 days and 10 days.
Results and discussion
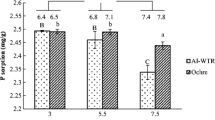
The maximum P sorption capacity of ochre, Al-based WTR, Fe-based WTR and Fe-CaO-based WTR estimated from the linearized Langmuir model following a 24-h equilibration period was 10.1, 13.7, 2.4 and 9.3 mg P g−1, respectively. However, extending the equilibration time from 24 h to 5 days increased the estimated maximum P sorption capacity for these IBPs by factors of 2.2, 2.1, 6.8 and 2.3, respectively. No significant increase was found in estimates of the maximum P sorption capacity when further extending the equilibration time to 10 days.
Conclusions
A minimum equilibration period of 5 days is recommended to avoid underestimating the maximum P sorption capacity of the IBPs examined in this paper. Each of the IBPs we evaluated was able to sorb P from solution, although with variable capacity (maximum sorption capacity after 5 days of equilibration ranged from 16.3–28.5 mg P g−1). These findings emphasise the importance of accurate quantification of the P sorption capacity of IBPs before application.




Similar content being viewed by others
References
Babatunde AO, Zhao YQ (2007) Constructive approaches toward water treatment works sludge management: an international review of beneficial reuses. Crit Rev Environ Sci Technol 37(2):129–164
Babatunde AO, Zhao YQ (2010) Equilibrium and kinetic analysis of phosphorus adsorption from aqueous solution using waste alum sludge. J Hazard Mater 184(1):746–752
Babatunde AO, Zhao YQ, Burke AM, Morris MA, Hanrahan JP (2009) Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ Pollut 157(10):2830–2836
Ballantine DJ, Tanner CC (2010) Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: a review. N Z J Agric Res 53(1):71–95
Barrow NJ (1978) The description of phosphate adsorption curves. J Soil Sci 29(4):447–462
Barrow NJ (2008) The description of sorption curves. Eur J Soil Sci 59(5):900–910
Bohn HL, McNeal BL, O'Connor GA (2001) Soil chemistry, 3rd edn. Wiley, New York
Bolster CH (2008) Revisiting a statistical shortcoming when fitting the Langmuir model to sorption data. J Environ Qual 37(5):1986–1992
Bolster CH, Hornberger GM (2007) On the use of linearized Langmuir equations. Soil Sci Soc Am J 71(6):1796–1806
Boujelben N, Bouzid J, Elouear Z, Feki M, Jamoussi F, Montiel A (2008) Phosphorus removal from aqueous solution using iron coated natural and engineered sorbents. J Hazard Mater 151(1):103–110
Bozika E (2001) Phosphorus removal from wastewater using sludge from mine drainage treatment settling ponds. MSc thesis, University of Edinburgh
Cucarella V, Renman G (2009) Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments—a comparative study. J Environ Qual 38(2):381–392
Dayton EA, Basta NT (2001) Characterization of drinking water treatment residuals for use as a soil substitute. Water Environ Res 73:52–57
Dayton EA, Basta NT (2005a) Use of drinking water treatment residuals as a potential best management practice to reduce phosphorus risk index scores. J Environ Qual 34(6):2112–2117
Dayton EA, Basta NT (2005b) A method for determining the phosphorus sorption capacity and amorphous aluminum of aluminum-based drinking water treatment residuals. J Environ Qual 34(3):1112–1118
Deasy C, Quinton JN, Silgram M, Bailey AP, Jackson B, Stevens CJ (2009) Mitigation options for sediment and phosphorus loss from winter-sown arable crops. J Environ Qual 38(5):2121–2130
Dorioz JM, Wang D, Poulenard J, Trevisan D (2006) The effect of grass buffer strips on phosphorus dynamics—a critical review and synthesis as a basis for application in agricultural landscapes in France. Agric Ecosyst Environ 117(1):4–21
Fenton O, Healy MG, Rodgers M, Huallachain DO (2009a) Site-specific P absorbency of ochre from acid mine-drainage near an abandoned Cu-S mine in the Avoca–Avonmore catchment, Ireland. Clay Miner 44(1):113–123
Fenton O, Healy MG, Rodgers M (2009b) Use of ochre from an abandoned metal mine in the south east of Ireland for phosphorus sequestration from dairy dirty water. J Environ Qual 38(3):1120–1125
Fox RL, Kamprath EJ (1970) Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Sci Soc Am Proc 34(6):902–907
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Soil Res 43(2):189–202
Heal KV, Younger PL, Smith KA, Glendinning S, Quinn P, Dobbie KE (2004) Removing phosphorus from sewage effluent and agricultural runoff using recovered ochre. In: Valsami-Jones E (ed) Phosphorus in Environmental Technology: Removal, Recovery and Applications. IWA Publishing, London, pp 321–335
Heal KV, Dobbie KE, Bozika E, McHaffie H, Simpson AE, Smith KA (2005) Enhancing phosphorus removal in constructed wetlands with ochre from mine drainage treatment. Water Sci Technol 51(9):275–282
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail DB (2007) Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic matter. Biol Fertil Soils 44(2):277–288
Ippolito JA, Barbarick KA, Heil DM, Chandler JP, Redente EF (2003) Phosphorus retention mechanisms of a water treatment residual. J Environ Qual 32(5):1857–1864
Ippolito JA, Barbarick KA, Elliott HA (2011) Drinking water treatment residuals: a review of recent uses. J Environ Qual 40(1):1–12
Kirkkala T, Ventelä AM, Tarvainen M (2012) Long-term field-scale experiment on using lime filters in an agricultural catchment. J Environ Qual 41(2):410–419
Kröger R, Lizotte RE, Douglas Shields F, Usborne E (2012) Inundation influences on bioavailability of phosphorus in managed wetland sediments in agricultural landscapes. J Environ Qual 41(2):604–614
Leader JW, Dunne EJ, Reddy KR (2008) Phosphorus sorbing materials: sorption dynamics and physicochemical characteristics. J Environ Qual 37(1):174–181
Maguire RO, Foy RH, Bailey JS, Sims JT (2001) Estimation of the phosphorus sorption capacity of acidic soils in Ireland. Eur J Soil Sci 52(3):479–487
Makris KC, El-Shall H, Harris WG, O'Connor GA, Obreza TA (2004a) Intraparticle phosphorus diffusion in a drinking water treatment residual at room temperature. J Colloid Interface Sci 277(2):417–423
Makris KC, Harris WG, O'Connor GA, Obreza TA (2004b) Phosphorus immobilization in micropores of drinking-water treatment residuals: implications for long-term stability. Environ Sci Technol 38(24):6590–6596
Makris KC, Harris WG, O'Connor GA, Obreza TA, Elliott HA (2005) Physicochemical properties related to long-term phosphorus retention by drinking-water treatment residuals. Environ Sci Technol 39(11):4280–4289
McGechan MB, Lewis DR (2002) SW—soil and water: sorption of phosphorus by soil, part 1: principles, equations and models. Biosyst Eng 82(1):1–24
McKeague JA, Day JH (1993) Ammonium oxalate extraction of amorphous iron and aluminium. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publ, Boca Raton, p 241
Nair PS, Logan TJ, Sharpley AN, Sommers LE, Tabatabai MA, Yuan TL (1984) Interlaboratory comparison of a standardized phosphorus adsorption procedure. J Environ Qual 13(4):591–595
O’Rourke SM, Foy RH, Watson CJ, Gordon A, Doody D (2012) Assessment of co‐blending water treatment residual with dairy manure to reduce phosphorus concentrations in run‐off in Northern Ireland. Soil use Manag 28(2):157–166
Ockenden MC, Deasy C, Quinton JN, Bailey AP, Surridge B, Stoate C (2012) Evaluation of field wetlands for mitigation of diffuse pollution from agriculture: sediment retention, cost and effectiveness. Environ Sci Policy 24:110–119
O'Connor GA, Elliott HA, Lu P (2002) Characterizing water treatment residuals phosphorus retention. Soil Crop Sci Soc Florida Proc 61:67–73
Oelkers EH, Hering JG, Zhu C (2011) Water: is there a global crisis? Elements 7(3):157–162
Penn CJ, McGrath JM, Rounds E, Fox G, Heeren D (2012) Trapping phosphorus in runoff with a phosphorus removal structure. J Environ Qual 41(3):672–679
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24(4):201–207
Staunton S, Nye PH (1989) The effect of non‐instantaneous exchange on the self‐diffusion of phosphate in soil. J Soil Sci 40(4):751–760
Ulén B, Bechmann M, Fölster J, Jarvie HP, Tunney H (2007) Agriculture as a phosphorus source for eutrophication in the north‐west European countries, Norway, Sweden, United Kingdom and Ireland: a review. Soil Use Manag 23(s1):5–15
Van der Zee SE, van Riemsdijk WH (1988) Model for long-term phosphate reaction kinetics in soil. J Environ Qual 17(1):35–41
Wagner DJ, Elliott HA, Brandt RC, Jaiswal D (2008) Managing biosolids runoff phosphorus using buffer strips enhanced with drinking water treatment residuals. J Environ Qual 37(4):1567–1574
Wang C, Wang Z, Lin L, Tian B, Pei Y (2012) Effect of low molecular weight organic acids on phosphorus adsorption by ferric-alum water treatment residuals. J Hazard Mater 203:145–150
Watts DB, Torbert HA (2009) Impact of gypsum applied to grass buffer strips on reducing soluble P in surface water runoff. J Environ Qual 38(4):1511–1517
Xu D, Xu J, Wu J, Muhammad A (2006) Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 63(2):344–352
Yang Y, Tomlinson D, Kennedy S, Zhao YQ (2006) Dewatered alum sludge: a potential adsorbent for phosphorus removal. Water Sci Technol 54:207–213
Acknowledgments
The authors would like to thank Ian Hotson from United Utilities, Stuart Widdowson from the UK Coal Authority and Joe Bartram from the Integrated Water Services (IWS) for sourcing and providing information on the by-products used in this study. We also thank Helen Quirk for the support in laboratory analyses. This research was supported by IDB Merit Scholarship Program for High Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Habibiandehkordi, R., Quinton, J.N. & Surridge, B.W.J. Effect of equilibration time on estimates of the maximum phosphorus sorption capacity of industrial by-products using the Langmuir model. J Soils Sediments 14, 1818–1828 (2014). https://doi.org/10.1007/s11368-014-0936-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0936-y