Abstract
Purpose
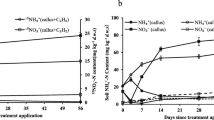
Nitrification is a key process in the global nitrogen cycle, of which the first and rate-limiting step is catalyzed by ammonia monooxygenase. Root cap cells are one of substrates for microorganisms that thrive in the rhizosphere. The degradation of root cap cells brings about nitrification following ammonification of organic nitrogen derived from the root cap cells. This study was designed to gain insights into the response of ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) to mineralized N from root cap cells and the composition of active bacterial and archaeal ammonia oxidizers in rice soil.
Materials and methods
Rice callus cells were used as a model for root cap cells, and unlabelled (12C) and 13C-labelled callus cells were allowed to decompose in aerobic soil microcosms. Real-time quantitative polymerase chain reaction (PCR), DNA-based stable isotope probing (SIP), and denaturing gradient gel electrophoresis (DGGE) were applied to determine the copy number of bacterial and archaeal amoA genes and the composition of active AOB and AOA.
Results and discussion
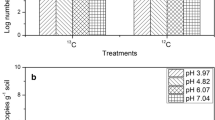
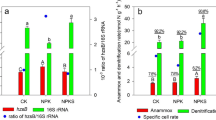
The growth of AOB was significantly stimulated by the addition of callus cells compared with the growth of AOA with a much lesser extent. AOB communities assimilated 13C derived from the callus cells, whereas no AOA communities grew on 13C-callus. Sequencing of the DGGE bands in the SIP experiments revealed that the AOB communities belonging to Nitrosospira spp. dominated microbial ammonia oxidation with rice callus amendment in soil.
Conclusions
The present study suggests that root cap cells of rice significantly stimulated the growth of AOB, and the active members dominating microbial ammonia oxidation belonged to Nitrosospira spp. in rice rhizosphere.






Similar content being viewed by others
References
Aleem MIH, Hoch GE, Varner JE (1965) Water as the source of oxidizing and reducing power in bacterial chemosynthesis. Proc Natl Acad Sci U S A 54:869–873
Avrahami S, Conrad R (2003) Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl Environ Microb 69:6152–6164
Bowatte S, Ishihara R, Asakawa S, Kimura M (2006a) Characterization of ammonia oxidizing bacteria associated with weeds in a Japanese paddy field using amoA gene fragments. Soil Sci Plant Nutr 52(5):593–600
Bowatte S, Jia Z, Ishihara R, Nakajima Y, Asakawa S, Kimura M (2006b) Molecular analysis of the ammonia oxidizing bacterial community in the surface soil layer of a Japanese paddy field. Soil Sci Plant Nutr 52(4):427–431
Bowatte S, Asakawa S, Okada M, Kobayashi K, Kimura M (2007) Effect of elevated atmospheric CO2 concentration on ammonia oxidizing bacteria communities inhabiting in rice roots. Soil Sci Plant Nutr 53:32–39
Cabrera ML, Kissel DE, Vigil MF (2005) Nitrogen mineralization from organic residues: research opportunities. J Environ Qual 34:75–79
Chen XP, Zhu YG, Xia Y, Shen JP, He JZ (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10:1978–1987
Clowes FAL (1976) Cell production by root caps. New Phytol 77:399–407
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammoniaoxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Gruber N, Galloway JN (2008) An earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hawes MC, Pueppke SG (1986) Sloughed peripheral root cap cells: yield from different species and callus formation from single cells. Am J Bot 73:1466–1473
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hetier JM (1986) Organic matter inputs to soil after growth of carbon-14–nitrogen-15 labeled maize. Soil Sci Soc Am J 50:76–80
Iijima M, Griffiths B, Bengough AG (2000) Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytol 145:477–482
Ikenaga M, Asakawa S, Muraoka Y, Kimura M (2003) Phylogenetic study on CTO primer-amplified ammonia-oxidizing bacteria and β-Proteobacteria associated with rice roots grown in a flooded paddy soil. Soil Sci Plant Nutr 49(5):719–727
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Keeneg DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL et al (eds) Methods of soil analysis: part 3. SSSA and ASA, Madison, pp 643–692
Koops HP, Pommerening-Röser A (2005) Genus III. Nitrosospira. In: Garrity GM (ed) Bergey’s manual of systematic bacteriology, 2nd edn., vol. 2. The Proteobacteria, part C: the alpha-, beta-, delta-, epsilonproteobacteria. Springer, New York, pp 868–869
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Ann Rev Microbiol 55:485–529
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Br Bioinforma 5:150–163
Lee CG, Watanabe T, Sato Y, Murase J, Asakawa S, Kimura M (2011) Bacterial populations assimilating carbon from 13C-labelled plant residue in soil: analysis by DNA-SIP approach. Soil Biol Biochem 43:814–822
Levičnik-Höfferle S, Nicol GW, Ausec L, Mulec I, Prosser JI (2012) Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not inorganic nitrogen. FEMS Microbiol Ecol 80:114–123
Li YL, Fan XL, Shen QR (2008) The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ 31:73–85
Li Y, Lee CG, Watanabe T, Murase J, Asakawa S, Kimura M (2011) Identification of microbial communities that assimilate substrate from root cap cells in an aerobic soil using a DNA-SIP approach. Soil Biol Biochem 43:1928–1935
Li Y, Watanabe T, Murase J, Asakawa S, Kimura M (2013) Identification of major capsid gene (g23) of T4-type bacteriophages that assimilate substrate from root cap cells in aerobic and anaerobic soil conditions applying a DNA-SIP approach. Soil Biol Biochem 63:97–105
Lueders T, Manefield M, Friedrich MW (2004) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol 6:73–78
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Morimoto S, Hayatsu M, Takada-Hoshino Y, Nagaoka K, Yamazaki M, Karasawa T, Takenaka M, Akiyama H (2011) Quantitative analyses of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in fields with different soil types. Microbes Environ 26:248–253
Murase J, Itoh K, Kano M, Kimura M (2003) Molecular analysis of β-Proteobacterial ammonia oxidizer populations in surface layers of a submerged paddy soil microcosm. Soil Sci Plant Nutr 49(6):909–913
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Nicolaisen MH, Ramsing NB (2002) Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J Microbiol Meth 50:189–203
Radajewski S, Webster G, Reay DS, Morris SA, Ineson P, Nedwell DB, Prosser JI, Murrell JC (2002) Identification of active methylotroph populations in an acidic forest soil by stable-isotope probing. Microbiol 148:2331–2342
Rolfe R, Meselson M (1959) The relative homogeneity of microbial DNA. Proc Natl Acad Sci U S A 45:1039–1043
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Sawatsky N, Soper RJ (1941) A quantitative measurement of the nitrogen loss from the root system of field peas (Pisum avense L.) grown in the soil. Soil Biol Biochem 23:255–259
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E, Streit W, Stahl DA, Wagner M, Schleper C (2010) Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol 18:331–340
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tian XF, Hu HW, Ding Q, Song MH, Xu XL, Zheng Y, Guo LD (2014) Influence of nitrogen fertilization on soil ammonia-oxidizers and denitrifiers abundance, microbial biomass and enzyme activities in an alpine meadow. Biol Fertil Soils 50(4):703–713
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidizing archaea and bacteria in soil microcosms. ISME J 5:1067–1071
Watanabe A, Kimura M (1999) Influence of chemical properties of soils on methane emission from rice paddies. Comm Soil Sci Plant Anal 30:2449–2463
Watanabe T, Asakawa S, Nakamura A, Nagaoka K, Kimura M (2004) DGGE method for analyzing 16S rDNA of methanogenic archaeal community in paddy field soil. FEMS Microbiol Lett 232:153–163
Watanabe T, Lee GC, Murase J, Asakawa S, Kimura M (2011) Carbon flow into ammonia-oxidizing bacteria and archaea during decomposition of 13C-labeled plant residues in soil. Soil Sci Plant Nutr 57:775–785
Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X, Zhu J, Xiong Z, Xu J, Cai Z, Jia Z (2011) Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5:1226–1236
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci U S A 107:17240–17245
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Acknowledgments
Yong Li extends his gratitude to the China Scholarship Council for their financial support in conducting this study. The authors also thank Professor Hongjie Di, Zhejiang University, for his insightful and helpful suggestions for improving the manuscript. This work is partially supported by the Natural Science Foundation of China (41301254), Specialized Research Fund for the Doctoral Program of Higher Education (20130101120182), Foundation of Zhejiang Educational Committee (Y201329798), and Fundamental Research Funds for the Central Universities (2014QNA6008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Comparison of DGGE banding patterns of 13C-enriched AOA community. N, 12 and 13 in the figure indicate no callus treatment, 12C-callus treatment and 13C-callus treatment, respectively; 4-11 indicate the fraction number (PPTX 445 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Watanabe, T., Murase, J. et al. Abundance and composition of ammonia oxidizers in response to degradation of root cap cells of rice in soil microcosms. J Soils Sediments 14, 1587–1598 (2014). https://doi.org/10.1007/s11368-014-0910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0910-8