Abstract
Purpose
Nitrous oxide (N2O) production and reduction rates are dependent on the interactions with each other and it is therefore important to evaluate them within the context of simultaneously operating N2O emission and reduction. The objective of this study was to quantify the simultaneously occurring N2O emission and reduction across a range of subtropical soils in China, to gain a mechanistic understanding of potential N2O dynamics under the denitrification condition and their important drivers, and to evaluate the potential role of the subtropical soils as either sources or sinks of N2O through denitrification.
Materials and methods
Soils (45, from a range of different land uses and soil parent materials) were collected from the subtropical region of Jiangxi Province, China, and tested for their potential capacity for N2O emission and N2O reduction to N2 during denitrification. N2O emission and reduction were determined in a closed system under N2 headspace after the soils were treated with 200 mg kg−1 NO −3 -N and incubation at 30 °C for 28 days. The soil physical and chemical properties, the temporal variations in headspace N2O concentration, and NO −3 -N and NH +4 -N concentrations in the soil slurry were measured.
Results and discussion
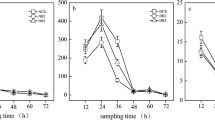
Variations in N2O concentration (N) over incubation time (t) were consistent with an equation in which average R 2 = 0.84 ± 0.11 (p < 0.05): \( N = A \times \left( {1 - \exp \left( { - {k_1} \times t} \right)} \right) - B \times \exp \left( {{k_2} \times t} \right) \), where A is the total N2O emission during the incubation, B is a constant, and k 1 and k 2 are the N2O emission constant and reduction constants, respectively. The results of the simulation showed that k 1 was greater than k 2. The reduced amount of NO −3 -N in the first 7 days of incubation and the N2O emission rate (the percentage of A value relative to the amount of NO −3 -N reduced during the 28-day incubation, R n) were able to explain 82.9 % (p < 0.01) of the variation in total N2O emission (A) during the incubation for the soil samples studied, indicating that the total amount of N2O emitted was determined predominately by denitrification capacity. Soil organic carbon content and soil nitrogen mineralization are the key factors that determine differences in the amounts of reduced NO −3 -N among the soil samples. The R n value decreased with increasing k 2 (p < 0.01), indicating that soils with higher N2O reduction capacity under these incubation conditions would emit less N2O per unit of denitrified NO −3 -N than the other soils. Results are valuable in the evaluation of net N2O emissions in the subtropical soils and the global N budget.
Conclusions
In a closed, anaerobic system, variations in N2O concentration in the headspace over the incubation time were found to be compatible with a nonlinear equation. Soil organic carbon and the amount of NH +4 -N mineralized from the organic N during the first 7 days of incubation are the key factors that determine differences in the N2O emission constant (k 1), the N2O reduction constant (k 2), the total N2O emission during the incubation (A) and the N2O emission rate (R n).



Similar content being viewed by others
References
Ahn YH (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721
Babić KH, Schauss K, Hai B, Sikora S, Redžepović S, Radl V, Schloter M (2008) Influence of different Sinorhizobium meliloti inocula on abundance of genes involved in nitrogen transformations in the rhizosphere of alfalfa (Medicago sativa L.). Environ Microbiol 10:2922–2930
Baggs EM (2011) Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr Opin Env Sust 3:321–327
Bandibas J, Vermoesen A, De Groot CJ, Van Cleemput O (1994) The effect of different moisture regimes and soil characteristics on nitrous oxide emission and consumption by different soils. Soil Sci 158:106–114
Bergstermann A, Cárdenas L, Bol R, Gilliam L, Goulding K, Meijide A, Scholefield D, Vallejo A, Well R (2011) Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol Biochem 43:240–250
Betlach MR, Tiedje JM (1981) Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol 42:1074–1084
Blackmer AM, Bremner JM (1978) Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol Biochem 10:189–191
Bouwman AF (1998) Nitrogen oxides and tropical agriculture. Nature 392:866–867
Bru D, Ramette A, Saby NPA, Dequiedt S, Ranjard L, Jolivet C, Arrouays D, Philippot L (2011) Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. ISME J 5(3):532–542
Cárdenas M, Hawkins JMB, Chadwick D, Scholefield D (2003) Biogenic gas emissions from soils measured using a new automated laboratory incubation system. Soil Biol Biochem 35:867–870
Cartaxana P, Lloyd D (1999) N2, N2O and O2 profiles in a Tagus Estuary Salt Marsh. Estuar Coast Shelf S 48:751–756
Chapuis-Lardy L, Wrage N, Metay A, Chotte JL, Bernoux M (2007) Soils, a sink for N2O? A review. Global Change Biol 13:1–17
Dendooven L, Splatt P, Anderson JM, Scholefield D (1994) Kinetics of the denitrification process in a soil under permanent pasture. Soil Biol Biochem 26:361–370
Driessen P, Deckers J, Nachtergaele F (2001) World Soil Sources Reports 94: Lecture Notes on the Major Soils of the World. FAO, Rome
Editorial Committee of ECA (2005) Encyclopedia of Chinese Agriculture, Jiangxi Volume. Chinese Press of Agriculture, Beijing
Ellis S, Dendooven L, Goulding KWT (1996) Quantitative assessment of soil nitrate disappearance and N2O evolution during denitrification. Soil Biol Biochem 28:589–595
Firestone MK, Firestone RB, Tiedje JM (1980) Nitrous oxide from soil denitrification: factors controlling its biological production. Science 208:749–751
Hack E, Bachmann G, Zechmeister-Boltenstern S (2004) Microbial nitrogen turnover in soils under different types of natural forest. Forest Ecol Manag 188:101–112
Hallin S, Jones CM, Schloter M, Philippot L (2009) Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J 3:597–605
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundance of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189
Henry S, Texier S, Hallet S et al (2008) Disentangling the rhizosphere effect on nitrate reducers and denitrifiers: insight into the role of root exudates. Environ Microbiol 10:3082–3092
Institute of Soil Science, Chinese Academy of Sciences (1978) Soil physical and chemical analysis. Shanghai Scientific and Technical Press, Beijing
Laverman AM, Zoomer HR, Verhoef HA (2001) The effect of oxygen, pH and organic carbon on soil-layer specific denitrifying capacity in acid coniferous forest. Soil Biol Biochem 33:683–687
Lu RK (2000) Soil agro-chemical analyses. Agricultural Technical Press of China, Beijing
Mathieu O, Lévêque J, Hénault C, Milloux MJ, Bizouard F, Andreux F (2006) Emissions and spatial variability of N2O, N2 and nitrous oxide mole fraction at the field scale, revealed with 15N isotopic techniques. Soil Biol Biochem 38:941–951
McLain JET, Martens DA (2006) N2O production by heterotrophic N transformations in a semiarid soil. Appl Soil Ecol 32:253–263
Mei LJ, Yang LZ, Wang DJ, Yin B, Hu J, Yin SX (2004) Nitrous oxide production and consumption in serially diluted soil suspensions as related to in situ N2O emission in submerged soils. Soil Biol Biochem 36:1057–1066
Morley N, Baggs EM (2010) Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol Biochem 42:1864–1871
Philippot L, Andert J, Jones CM, Bru D, Hallinz S (2011) Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Global Change Biol 17:1497–1504
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Seufert G, Butterbach-Bahl K (2006) N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences 3:121–133
Rütting T, Huygens D, Müller C, van Cleemput O, Godoy R, Boeckx P (2008) Functional role of DNRA and nitrite reduction in a pristine south Chilean Nothofagus forest. Biogeochemistry 90:243–258
Senga Y, Mochida K, Fukumori R, Okamoto N, Seike Y (2006) N2O accumulation in estuarine and coastal sediments: the influence of H2S on dissimilatory nitrate reduction. Estuar Coast Shelf S 67:231–238
Šimek M, Elhottová D, Klimeš F, Hopkins DW (2004) Emissions of N2O and CO2, denitrification measurements and soil properties in red clover and ryegrass stands. Soil Biol Biochem 36:9–21
Stein LY, Yung XL (2003) Production, isotopic composition, and atmospheric fate of biologically produced nitrous oxide. Annu Rev Earth Pl S 31:329–356
Stevens RJ, Laughlin RJ, Malone JP (1998) Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol Biochem 30:1119–1126
Tiedje JM (1982) Denitrification, methods of soil analysis. American Society of Agronomy, Madison, pp 1011–1026
Toyoda S, Mutobe H, Yamagishi H, Yoshida N, Tanji Y (2005) Fractionation of N2O isotopomers during production by denitrifiers. Soil Biol Biochem 37:1535–1545
Trogler WC (1999) Physical properties and mechanisms of formation of nitrous oxide. Coord Chem Rev 187:303–327
Vallejo A, Skiba UM, García-Torres L, Arce A, López-Fernández S, Sánchez-Martín L (2006) Nitrogen oxides emission from soils bearing a potato crop as influenced by fertilization with treated pig slurries and composts. Soil Biol Biochem 38:2782–2793
Xu YB, Cai ZC (2007) Denitrification characteristics of subtropical soils in China affected by soil parent material and land use. Eur J Soil Sci 58:1293–1303
Xu RK, Zhao AZ, Li QM, Kong XL, Ji GL (2003) Acidity regime of the Red Soils in a subtropical region of southern China under field conditions. Geoderma 115:75–84
Zhang JB, Zhu TB, Cai ZC, Müller C (2011) Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 342:419–432
Zhao QG, Xie WM, He XY, Wang MZ (1988) Red soils in Jiangxi. Science and Technology Press of Jiangxi, Nanchang
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosys 63:117–127
Acknowledgments
The project was financially supported by the National Natural Science Foundation of China (31101605), the Research Foundation of State Key Laboratory of Soil and Sustainable Agriculture, Chinese Academy of Sciences (0812000051) and the Natural Science Foundation of Yunnan Province (2010ZC083). We are grateful to Dr. W. Zhao for analyzing total soil N, total soil C, and clay content. Appreciation is also extended to Prof. H. Xu for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Xu, Y., Cai, Z. & Xu, Z. Production and consumption of N2O during denitrification in subtropical soils of China. J Soils Sediments 12, 1339–1349 (2012). https://doi.org/10.1007/s11368-012-0548-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0548-3