Abstract
Purpose
Sono-Fenton-like process was tested to degrade naphthalene in spiked soil utilizing mineral iron as a catalyst to generate radical species as well as to evaluate the efficiency of the proposed method and to optimize the treatment conditions. The study assessed the relationships between the most significant process variables (initial naphthalene and hydrogen peroxide concentrations) and their response on naphthalene degradation efficiency using central composite design at various ultrasound irradiation intensities. The electrical energy per order was also calculated to illustrate economic benefits of this “hybrid” technique.
Materials and methods
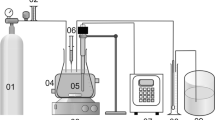
Sono-oxidation was performed with naphthalene contaminated soil (200, 400, and 800 mg kg−1 dry weight) to mimic industrially contaminated soil conditions in a closed Pyrex glass reactor in the presence of naturally occuring mineral iron, various ultrasound irradiations (100, 200, and 400 W), and hydrogen peroxide concentrations (100, 200, and 600 mg L−1). Naphthalene samples were analyzed by gas chromatography–mass spectometry using an Agilent 6890 N chromatograph coupled to a quadrupole Agilent 5975 inert XL mass selective spectrometer. Easily exchangeable iron was extracted by citrate–bicarbonate–dithionite extraction, and total iron was extracted by acid digestion and were analyzed using an atomic absorption spectroscopy flame (Perkin-Elmer 300). The surface morphology of the soil sample was observed by scanning electron microscopy, and the specific surface area was determined using Brunauer–Emmett–Teller method on a Quantachrome Autosorb 1 analyzer. The STATISTICA 7 software was used for regression and graphical analyses of the data.
Results and discussion
Scanning electron microscopy revealed that soil was predominantly associated with quartz and coarse and fine sand fractions and contained a significant amount of iron (7.1 g kg−1 of total and 2.3 g kg−1 of easily exchangeable iron). Control experiments showed that, in the absence of hydrogen peroxide, naphthalene degradation up to 35% was achieved, which suggested that mineral iron was able to catalyze the production of hydroxyl radicals when ultrasound irradiation was used as an oxidizing agent. The optimum values (600 mg L−1 of hydrogen peroxide and 200 mg kg−1 of naphthalene concentrations) were obtained by solving the regression equations. Various kinetic constants such as kN, kN/OH*, ksurf, half-life (τ1/2), and [OH*]SS were calculated for sono-Fenton-like process. The addition of hydrogen peroxide demonstrated nearly tenfold decrease in electrical energy per order or in electrical costs from 2,063–4,210 kWh ton-order−1 and 144.4–294.7 € ton−1 at 0 mg L–1 H2O2 to 26.4–42 kWh ton-order−1 in comparison to 1.7–2.9 € ton−1 at 600 mg L−1 H2O2 addition to the system.
Conclusions
Mineral iron was able to catalyze the degradation of naphthalene in the presence of ultrasound (up to 78% at 100 W and 97% at 400 W) and various concentrations of hydrogen peroxide. The optimum values (600 mg L−1 of hydrogen peroxide and 200 mg kg1 of naphthalene concentrations at 200 and 400 W) indicated up to a maximum 97% reduction in naphthalene concentration in soil after 2 h of treatment. The Fenton-like oxidation of naphthalene in the presence of ultrasound has a potential to be used for practical purposes, however, it warrants further research regarding the use of industrially contaminated soil with multiple organic contaminants. Moreover, improvement in the reactor design is necessary to prolong the lifetime of the sonotrode which tends to wear off due to ultrasound waves that propagates back to the sonotrode from the walls and the bottom of the reactor. Despite the current difficulties, ultrasound has a bright future in on-site soil remediation field and may become one of the most feasible options and environmentally sound techniques.



Similar content being viewed by others
References
Adewuyi YG (2005) Sonochemistry in environmental remediation. 1. Combinative and hybrid sonophotochemical oxidation Processes for the treatment of pollutants in water. Environ Sci Technol 39:3409–3420. doi:10.1021/es049138y
Adewuyi YG, Appaw C (2002) Sonochemical oxidation of carbon disulfide in aqueous solutions: reaction kinetics and pathways. Indust Eng Chem Res 41:4957–4964. doi:10.1021/ie020069a
Ahmad AL, Ismail S, Bhatia S (2005) Optimization of coagulation-flocculation process for palm oil mill effluent using response surface methodology. Environ Sci Technol 39:2828–2834. doi:10.1021/es0498080
Aleboyeh A, Olya ME, Aleboyeh H (2008) Electrical energy determination for an azo dye decolorization and mineralization by UV/H2O2 advanced oxidation process. Chem Eng J 137:518–524
Alshawabkeh AN, Sarahney H (2005) Effect of current density on enhanced transformation of naphthalene. Environ Sci Technol 39:5837–5843. doi:10.1021/es049645f
Bolton JR, Bircher KG, Tumas W, Tolman CA (2001) Figures-of-merit for the technical development and application of advanced technologies for both electric- and solar-driven systems. Pure Appl Chem 73:627–637. doi:10.1351/pac200173040627
Brodowski S, Amelung W, Haumaier L, Abetz C, Zech W (2005) Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 128:116–129
Catalkaya EC, Kargi F (2007) Effects of operating parameters on advanced oxidation of diuron by the Fenton's reagent: a statistical design approach. Chemosphere 69:485–492
Christensen BT (1996) Carbon in primary and secondary organo-mineral complexes. In: Carter MR, Stewart BA (eds) Advances in soil sciences: structures and organic matter storage in agricultural soils. CRC Press, Boca Raton, Florida, pp 97–165
ECB (2003) European Union Risk Assessment report: naphthalene. In: Munn SJ et al (eds) Joint Research Centre, Institute for Health and Consumer Protection, Luxembourg: European Commission
Entezari MH, Pétrier C (2004) A combination of ultrasound and oxidative enzyme: sono-biodegradation of phenol. Appl Catal B- Environ 53:257–263
EPA (1982) Determination of Polynuclear Aromatic Hydrocarbons in Industrial and Municipal Wastewaters, National Technical Information Service, PB82-258799, Springfield, Virginia, USA
Florian D, Barnes RM, Knapp G (1998) Comparison of microwave-assisted acid leaching techniques for the determination of heavy metals in sediments, soils, and sludges. J Anal Chem 362:558–565. doi:10.1007/s002160051124
Ghasempur S, Torabi S-F, Ranaei-Siadat S-O, Jalali-Heravi M, Ghaemi N, Khajeh K (2007) Optimization of peroxidase-catalyzed oxidative coupling process for phenol removal from wastewater using response surface methodology. Environ Sci Technol 41:7073–7079. doi:10.1021/es070626q
Guo Z, Zheng Z, Zheng S, Hu W, Feng R (2005) Effect of various sono-oxidation parameters on the removal of aqueous 2, 4-dinitrophenol. Ultrason Sonochem 12:461–465
Hagenson LC, Doraiswamy LK (1998) Comparison of the effects of ultrasound and mechanical agitation on a reacting solid-liquid system. Chem Eng Sci 53:131–148
Hawthorne SB, Grabanski CB, Martin E, Miller DJ (2000) Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J Chromatogr A 892:421–433
Hill T, Lewicki P (2007) STATISTICS methods and applications. Statsoft, Oklahoma, USA
Hunt CP, Singer MJ, Kletetschka G, TenPas J, Verosub KL (1995) Effect of citrate-bicarbonate-dithionite treatment on fine-grained magnetite and maghemite. Earth Planet Sci Lett 130:87–94. doi:10.1016/0012-821X(94)00256-X
Ince NH, Tezcanli G, Belen RK, Apikyan IG (2001) Ultrasound as a catalyzer of aqueous reaction systems: the state of the art and environmental applications. Appl Catal B-Environ 29:167–176
Keck A, Gilbert E, Köster R (2002) Influence of particles on sonochemical reactions in aqueous solutions. Ultrasonics 40:661–665. doi:10.1016/S0041-624X(02)00195-6
Kim YU, Wang MC (2003) Effect of ultrasound on oil removal from soils. Ultrasonics 41:539–542. doi:10.1016/S0041-624X(03)00168-9
Kim JK, Martinez F, Metcalfe IS (2007) The beneficial role of use of ultrasound in heterogeneous Fenton-like system over supported copper catalysts for degradation of p-chlorophenol. Catal Today 124:224–231
Koivula MP, Kujala K, Rönkkömäki H, Mäkelä M (2009) Sorption of Pb(II), Cr(III), Cu(II), As(III) to peat, and utilization of the sorption properties in industrial waste landfill hydraulic barrier layers. J Hazard Mater 164:345–352. doi:10.1016/j.jhazmat.2008.08.008
Lair A, Ferronato C, Chovelon J-M, Herrmann J-M (2008) Naphthalene degradation in water by heterogeneous photocatalysis: an investigation of the influence of inorganic anions. J Photoch Photobio A 193:193–203
Landfester K (2001) The generation of nanoparticles in miniemulsions. Wiley, New York
Liang J, Komarov S, Hayashi N, Kasai E (2007) Improvement in sonochemical degradation of 4-chlorophenol by combined use of Fenton-like reagents. Ultrason Sonochem 14:201–207
Lin JG, Ma YS (2000) Oxidation of 2-chlorophenol in water by ultrasonic/Fenton method. J Environ Eng-ASCE 126:130–137. doi:10.1061/(ASCE)0733-9372(2000)126:2(130)
Lin J-j, X-s Z, Liu D, Z-g Yu, Zhang Y, Xu H (2008) The decoloration and mineralization of azo dye C.I. Acid Red 14 by sonochemical process: rate improvement via Fenton's reactions. J Hazard Mater 157:541–546
Liou M-J, Lu M-C (2008) Catalytic degradation of explosives with goethite and hydrogen peroxide. J Hazard Mater 151:540–546
Lu M-C (2000) Oxidation of chlorophenols with hydrogen peroxide in the presence of goethite. Chemosphere 40:125–130
Lu Y, Weavers LK (2002) Sonochemical desorption and destruction of 4-chlorobiphenyl from synthetic sediments. Environ Sci Technol 36:232–237. doi:10.1021/es010641+
Mascolo G, Ciannarella R, Balest L, Lopez A (2008) Effectiveness of UV-based advanced oxidation processes for the remediation of hydrocarbon pollution in the groundwater: a laboratory investigation. J Hazard Mater 152:1138–1145
Mason TJ, Lorimer PJ (2002) Applied sonochemistry: uses of power ultrasound in chemistry and processing. Wiley, New York
Mason TJ, Collings A, Sumel A (2004) Sonic and ultrasonic removal of chemical contaminants from soil in the laboratory and on a large scale. Ultrason Sonochem 11:205–210
Matta R, Hanna K, Chiron S (2007) Fenton-like oxidation of 2, 4, 6-trinitrotoluene using different iron minerals. Sci Total Environ 385:242–251
Meegoda JN, Perera R (2001) Ultrasound to decontaminate heavy metals in dredged sediments. J Hazard Mater 85:73–89. doi:10.1016/S0304-3894(01)00222-9
Neppolian B, Jung H, Choi H, Lee JH, Kang J-W (2002) Sonolytic degradation of methyl tert-butyl ether: the role of coupled Fenton process and persulphate ion. Water Res 36:4699–4708
Nystroem GM, Ottosen LM, Villumsen A (2005) Electrodialytic removal of Cu, Zn, Pb and Cd from harbor sediment: influence of changing experimental conditions. Environ Sci Technol 39:2906–2911. doi:10.1021/es048930w
Oturan MA, Brillas E (2007) Electrochemical advanced oxidation processes (EAOPs) for environmental applications. Portugaliae Electrochim Acta 25:1–18
Pham TD, Shrestha RA, Virkutyte J, Sillanpää M (2009) Combined ultrasonication and electrokinetic remediation for persistent organic removal from contaminated kaolin. Electrochim Acta 54:1403–1407
Rehorek A, Tauber M, Gübitz G (2004) Application of power ultrasound for azo dye degradation. Ultrason Sonochem 11:177–182
Rokhina EV, Lahtinen M, Nolte MCM, Virkutyte J (2009) The influence of ultrasound on the RuI3-catalyzed oxidation of phenol: catalyst study and experimental design. Appl Catal B-Environ 87:162–170
Saichek RE, Reddy K (2005) Electrokinetically enhanced remediation of hydrophobic organic compounds in soils: a review. Crit Rev Env Sci Tec 35:115–192. doi:10.1080/10643380590900237
Sandoval-González A, Silva-Martínez S, Blass-Amador G (2007) Ultrasound leaching and electrochemical treatment combined for lead removal soil. J New Mat Electrochem Systems 10:195–199
Stegmann R (2001) Treatment of contaminated soils: fundamentals, analysis, applications. Springer Verlag, Berlin
Stroud JL, Paton GI, Semple KT (2007) Importance of chemical structure on the development of hydrocarbon catabolism in soil. FEMS Microbiol Lett 272:120–126. doi:10.1111/j.1574-6968.2007.00750.x
Thangavadivel K, Megharaj M, Smart RSC, Lesniewski PJ, Naidu R (2009) Application of high frequency ultrasound in the destruction of DDT in contaminated sand and water. J Hazard Mater 168:1380–1386. doi:10.1016/j.jhazmat.2009.03.024
Tyre BW, Watts BJ, Miller GC (1991) Treatment of four biorefractory contaminants in soils using catalyzed hydrogen peroxide. J Environ Qual 20:832–838
Wang Y, Liu CS, Li FB, Liu CP, Liang JB (2009) Photodegradation of polycyclic aromatic hydrocarbon pyrene by iron oxide in solid phase. J Hazard Mater 162:716–723
Wiewióra A, Pérez-Rodrķguez JL, Perez-Maqueda LA, Drapala J (2003) Particle size distribution in sonicated high- and low-charge vermiculites. Appl Clay Sci 24:51–58
Yang GCC, Liu C-Y (2001) Remediation of TCE contaminated soils by in situ EK-Fenton process. J Hazard Mater 85:317–331
Yuan G, Soma M, Seyama H, Theng BKG, Lavkulich LM, Takamatsu T (1998) Assessing the surface composition of soil particles from some podzolic soils by X-ray photoelectron spectroscopy. Geoderma 86:169–181
Acknowledgments
Financial support from Finnish Academy (decision number 212649) and FP 6 Marie Curie European Re-integration Grants (ESF/2004/2.5.0-03-402/BPD-173/1) is highly acknowledged. Mathias Nolte from Technical University Hamburg-Harburg (Germany) is thanked for SEM measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaakko Paasivirta
Rights and permissions
About this article
Cite this article
Virkutyte, J., Vičkačkaite, V. & Padarauskas, A. Sono-oxidation of soils: degradation of naphthalene by sono-Fenton-like process. J Soils Sediments 10, 526–536 (2010). https://doi.org/10.1007/s11368-009-0153-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0153-2