Abstract
Purpose
The EU environmental footprint (EF) is a life cycle assessment (LCA)-based method which aims at assessing the environmental impacts of products and organisations through 16 midpoint impact categories, among which three address toxicity-related impacts. This paper presents the principles underpinning the calculation of the set of characterisation factors (CFs) for the toxicity-related impact categories in the EF version 3.0: freshwater ecotoxicity (ECOTOX), human toxicity cancer (HTOX_c) and human toxicity non-cancer (HTOX_nc).
Methods
In order to respond to the issues that emerged during the EF pilot phase, the input data and the calculation principles of the USEtox® model were updated. In particular, (i) robustness factors (RFs) were introduced to reduce the dominance of metals and to balance the lackness of a robust fate modelling for non-organic compounds in USEtox®; (ii) high-quality data were selected from databases of EU agencies (European Chemicals Agency and European Food Safety Authority) to guarantee the transparency and the reliability of input data; and (iii) a new approach based on HC20 (hazard concentration killing 20% of the exposed population) was implemented to derive freshwater ecotoxicity effect factors (EfF).
Results and discussion
The new approach increased the number of characterised chemicals in the three impact categories: ECOTOX (6038 chemicals, + 140%), HTOX_c (1024 chemicals, + 70%) and HTOX_nc (3317 chemicals, + 660%). Moreover, specific derivation principles were defined for assigning CFs also to relevant groups of chemicals (e.g. polycyclic aromatic hydrocarbons), and specific strategies were implemented to better align LCA toxicity data with data used for risk assessment purposes.
Conclusions
The new set of CFs was calculated to ensure a broader coverage of characterised chemicals and to overcome some limitations of the USEtox® model identified during the environmental footprint pilot phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The product and organisation environmental footprint (PEF and OEF, respectively) is a life cycle assessment-based method to assess the environmental performance of products and organisations (EC 2013a, b). The development of the PEF and OEF methods are part of the Single Market for Green Products Initiative that aims to establish a common and agreed method for assessing the environmental performance through the life cycle (EC 2013a, b and EC, 2021) and are now under discussion as reference methods for the Green Claims Initiative (EC, 2021a) and as method in support to the chemical strategy for sustainability (EC 2020) and the zero pollution action plan (EC 2021b). The PEF and OEF methods have been developed since 2013 by the Joint Research Centre of the European Commission (EC-JRC) in collaboration with DG Environment, involving stakeholders along the process. The methods were adopted by the European Commission in 2013 (EC, 2013b) the Recommendation 2021/9332/EU (EC, 2021), on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations.
Based on Life Cycle Assessment (LCA) (ISO 2006a, b), the PEF and OEF aim to quantify the expected environmental impacts of the life cycle of products and organisations by considering all the inputs (e.g. resource use) and outputs (e.g. emissions to the ecosphere) along the different life cycle stages (e.g. raw materials extraction, manufacturing, transport, use, etc.). For the PEF and OEF implementation, the environmental footprint (EF) method included recommendations for Life Cycle Impact Assessment (LCIA) models, addressing 16 impact categories, such as climate change and eutrophication. The comprehensive and systematic LCA-based method prevents trade-offs of environmental impacts between life cycle stages or impact categories (Sala et al. 2016). Three toxicity-related impact categories are assessed in the EF LCIA method: freshwater ecotoxicity (ECOTOX), human toxicity cancer (HTOX_c) and human toxicity non-cancer (HTOX_nc).
Within the LCA community, the assessment of toxicity impacts has been subject to intense development for more than two decades. The multimedia fate and effect model USEtox® (Rosembaum et al. 2008) has been developed as an international joint effort of various method developers, under the auspices of the United Nation Environmental Program and SETAC Life Cycle InitiativeFootnote 1 (UNEP-SETAC LCI). The model calculates the potential impact of chemicals on ecosystems and human health. USEtox® was launched as a consensus models in order to overcome the intrinsic differences in previously available models, such as CalTOX (McKone and Enoch 2002), USES-LCA (Huijbregts et al. 2001; Van Zelm et al. 2009), TRACI (Bare 2011), IMPACT 2002 + (Jolliet et al. 2003), EDIP 2003 (Hauschild & Potting, 2005) or MEEuP (Kemna et al. 2005), which generated toxicity impact results spanning over a few orders of magnitude (Hauschild et al. 2008; Rosenbaum et al. 2008). The European Commission recommended the USEtox® model to calculate the potential impact of chemical emissions for PEF and OEF (EC 2013a, b). During the EF pilot phase (2013-2018), involving 25 pilots in different sectors, the USEtox® model was systematically tested unveiling a number of limitations in relation to characterised results (Saouter et al. 2017a, b). The main limitations identified by stakeholders participating in the PEF pilots were: (i) metals always dominate toxicity scores and, in general, the model was not considered fitting the purpose for assessing the impacts of metals; (ii) physicochemical input data were not considered reliable for many chemicals; and (iii) ecotoxicity data were not aligned with those used for risk assessment purposes leading to potential inconsistencies in product evaluation when using different assessment schemes. This led to a temporary exclusion of toxicity among the PEF impact categories of the method.
To address these concerns, EC-JRC decided to improve some methodological aspects of the model, e.g. in terms of input data selection and calculation principles underpinning the characterisation factors (CFs). The refinement entailed: (i) a review of available options to improve exposure modelling and toxicity assessment of substances (Saouter et al. 2017a, b); (ii) the involvement of stakeholders through a workshop organised by the European Commission to collect feedbacks and suggestions towards an agreement on data selection procedures; and (iii) the collection and systematisation of available data from specific sources.
In particular, EC-JRC proposed: (i) a selection workflow for the identification of highly reliable data; (ii) the adoption of a more conservative approach to align the freshwater ecotoxicity data to those used for risk assessment (Saouter et al. 2019a, b), agreed during the Pellston workshop in 2018 (Owsianiak et al. 2019); and (iii) the introduction of robustness factors (RFs) to consider the uncertainty of the multimedia modelling for non-organic chemicals and to balance the contribution of different types of substances (i.e. metals, organics and inorganics). This set of CFs was adopted in the EF version 3.0 method (EC-JRC 2018; Fazio et al. 2018). All the details of the data selection and calculation are available in Saouter et al. (2018).
Building on these developments, the aim of this paper is to describe the process by which we derived the set of CFs for toxicity impact categories of the EF 3.0 method (i.e. ECOTOX, HTOX_c, HTOX_nc) and to analyse how the new impact indicators compare to the USEtox® model and how the limitations highlighted during the EF pilot phase were overcome. Implications for future development in the domain of toxicity characterisation are also reported.
2 Materials and methods
This section describes data sources and derivation principles used to obtain CFs for ECOTOX, HTOX_c and HTOX_nc. Hereinafter, all steps performed to ensure high-quality level of input data and results are reported: from raw physicochemical properties to eco- and human-toxicological data. Moreover, the comparison between the newly calculated CFs and those available in USEtox® is reported.
2.1 Toxicity-related characterisation factors (CFs)
For toxicity-related impact categories, CFs quantify the potential toxicological impact of chemicals on human health and freshwater ecosystems. They result from three substance-specific factors Eq. (1) (Rosenbaum et al. 2008):
-
Fate factor (FF), which refers to the distribution of the chemical in the environment. It is calculated for different environmental compartments (soil, air and water) and related sub-compartments at steady state.
-
Exposure factor (XF), which represents the bioavailable fraction of the chemical that may reach organisms in the ecosphere.
-
Effect factor (EfF), which refers to the intrinsic toxicity potential of the chemical.
$$CF=iF \times EfF$$(2)
In human exposure assessment, FFs and XFs are combined in the intake fraction (\(iF=FF\times XF\)), which describes the fraction of emission that is taken in by the overall exposed population (Rosenbaum et al. 2008). Hence, CFs are calculated as per Eq. (2).
In this study, USEtox® 2.1 (Fantke et al. 2017; USEtox 2018) is the model taken as starting point for the calculation improvements and for which the comparison are further described.
2.2 Data sources
In EF 3.0, new data sources were employed to calculate the CFs for ECOTOX, HTOX_c and HTOX_nc indicators. The aims of selecting new data sources were: (i) ensuring traceability of input data; (ii) improving data governance (i.e. having transparently reported the responsibility for data provision); and (iii) capitalising knowledge on data quality, towards the highest standards in selection of input data.
Three different substance properties databases were used to generate input data for the USEtox® 2.1 model: the database resulting from the implementation of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH-DB), the OpenFoodTox Database (OFT-DB) and the Pesticide Property Database (PPDB). The first two were provided by EU agencies: the European Chemical Agency (ECHA) and the European Food Safety Agency (EFSA), which are, respectively, the result of REACH regulation (EC 2006) and of the general food law regulation (EC 2002). The PPDB was developed by the Agriculture & Environment Research Unit (AERU) at the University of Hertfordshire. Its online version is the result of 20 years effort to collect and format pesticide data to be used freely for conducting substance risk assessment (Lewis et al. 2016).
The REACH-DB contains a large number of chemicals’ physico-chemical properties and toxicological data with different levels of quality, completeness and reliability. Thus, a proper data selection prior to its use is strongly recommended. OFT-DB holds quality-assured summary hazard data from EFSA’s substance risk assessments in food and feed (Dorne et al. 2017; S-IN 2015; Bassan et al. 2018). Contrary to REACH-DB, OFT-DB contains only data that are directly relevant for use in environmental and human risk assessment. PPDB contains selected quality assessed data on pesticides including physicochemical, toxicological, ecotoxicological, human health and other related data (Lewis et al. 2016; PPDB 2017).
In particular cases, substances were included in USEtox® 2.1 list and missing in the abovementioned data sources, for instance, substances which are banned in EU but still used in other part of the world (and so possibly appearing in life cycle inventories referring to stage of production outside EU). If not available elsewhere, input data originally included in the USEtox® 2.1 database were retained.
2.3 Physico-chemical properties to calculate fate and exposure factors
Physico-chemical properties of chemicals are used to calculate FFs and XFs. The USEtox® model requires several physico-chemical parameters. Nine of these parameters are mandatory for organic substances (molecular weight, acid dissociation, octanol–water partition coefficient, water solubility, vapour pressure, and degradation in water, sediment, soil and air), with three additional ones for inorganic and metal compounds (partition coefficient between water and suspended solids, suspended particles and soil particles) (Fantke et al. 2017).
Most of these parameters were retrieved from REACH-DB. The selection and database curation process aims to obtain a single data point by parameter and by chemical; it is described in detail in Saouter et al. (2018). The main challenge was to obtain the values with the highest quality, while keeping the procedure simple, reproducible and easy to understand. All data treatment and calculation were performed using the RStudio software (RStudio Team 2016) (code available in Saouter et al. (2018)).
2.3.1 Degradation rates
For calculating FFs, USEtox® requires degradation rates (s-1) in water, sediments, soil and air. The REACH–DB contains biodegradation data in wastewater treatment, surface water, sediment and soil as well as hydrolysis data for few inorganic compounds. When no half-life values were available, they were estimated from the biodegradability screening tests. A default degradation rate was assigned to each harmonised biodegradation category: “Readily biodegradable”, “Readily, but failing the 10 days window”, “Inherently biodegradable” and “Not readily biodegradable” according to the EU Technical Guidance Document on risk assessment (TGD) (EC-JRC 2003). For the “not readily biodegradable” category, the proposed TGD rate of 0 s-1 was replaced by the “recalcitrant” category of Biowin3 (US-EPA 2012), which is the EPISuite tool to estimate complete degradation rate of chemicals in water. As suggested in EPISuite (US-EPA 2012), division factors of 2 and 9 were applied to extrapolate biodegradation rates for sediment and soil from water degradation rate, respectively. Further analysis on extrapolation ratios for degradation rates in soil and sediments will be needed to increase data robustness.
2.3.2 Data gap filling and properties not available in REACH-DB
Physico-chemical data of REACH-DB were complemented, where needed, with OECD toolbox (Wegmann et al. 2009) and EPISuite (US-EPA 2018) data. These two tools were designed to estimate physico-chemical properties of chemicals, aiming at supporting procedures of assessing hazard of chemicals by filling data gaps. Regarding Henry’s law constant, missing values were calculated as ratio between vapour pressure and water solubility. PPDB (Lewis et al. 2016; PPDB 2017) and USEtox® 2.1 were consulted as last options. Degradation rate in air and bioaccumulation factor for fish (missing in REACH-DB) were estimated from EPISuite, following the procedure described in USEtox® (Fantke et al. 2017). Acid and basic dissociation constants were retrieved using ADMET® Predictor (SimulationsPlus 2016), which includes estimation models for substance ionisation.
2.3.3 Quality assessment of physico-chemical properties
Quality scores for physico-chemical properties (QSpc) were assigned to each parameter for each chemical in order to provide information on the reliability of data and the sources thereof. For each chemical and property, data with different quality levels could be retrieved. Only the data with the highest QSpc (or their geometric mean when more than one data was available) were retained.
Data quality reflects the completeness of data and it was assessed differently according to the characteristic of the sources, addressing the level of details of sources. For example, REACH data are the richest in terms of information; therefore, it allows to verify the alignment of testing conditions with experimental requirement. On the contrary, tools used for data gap filling provide no additional details, limiting the quality evaluation. QSpc are intended to valorise physico-chemical properties measured in standard experimental conditions (available in REACH-DB) than values resulting from data gap filling procedure (from OECD Toolbox, EPISuite and USEtox 2.1®). QSpc represent the priority list for data selection (Table 1).
From REACH-DB, data recorded as “key value for safety assessment” in the endpoint study summary in the ECHA dissemination website were always selected as first choice. These were considered the best option for EF purpose, as they are used in priority for safety assessment and hazard classification under REACH. For the remaining data in REACH-DB, a quality score (high, intermediate and low) was associated to each final physicochemical value. Highest scores were assigned when satisfying the following criteria: (i) experimental data were preferred over QSAR (quantitative structure–activity relationship) or read-across, (ii) experimental data obtained within the domain of application of the analytical methods (temperature, pH, etc.), (iii) data obtained being compliant with good laboratory practices (GLP) and (iv) data reported as an exact number (neither the instrument detection limit nor the method detection limits, labelled in the database with “>” or “<”) (Table SM1).
The QSpc of the data retrieved using data gap filling is related either to the source (PPDB, USEtox®, experimental value from EPISuite or OECD toolbox) or whether the chemical falls within the applicability domain (AD) of the estimation model. Predictions are not considered not reliable for chemicals outside AD.
2.4 Toxicological data and calculation principles to derive freshwater ecotoxicity effect factors
Ecotoxicity effect factors were calculated based on selected data sources and on a number of methodological choices described in the next sections.
2.4.1 Development of an ecotoxicity database
The three databases used to retrieve ecotoxicity data (REACH-DB, OFD-DB and PPDB) required different levels of data curation, namely preparation and treatment of data. The REACH-DB contained initially 305068 ecotoxicity records for 7714 substances. A selection procedure (Saouter et al. 2018, 2019a) was applied to select only the data deemed trustworthy enough to calculate effect factors. Selection criteria were based on: (i) reliability, evaluated by Klimish scores (Klimisch et al 1997); (ii) adequacy of the underpinning study; (iii) water media (i.e. freshwater); (iv) endpoint type, prioritising chronic effect values; (v) biological effects (e.g. growth or biomass for algae); (vi) reference point (e.g. EC10); (vii) duration of the experiment, according to endpoint and species; (viii) concentration assessment (i.e. measured or nominal); and (ix) replicates and testing conditions different from protocols. This led to the exclusion of about 82% of the initial dataset. OFT-DB contains only quality-assured data, thereby requiring fewer interventions to assure its compatibility with REACH-DB data (e.g. unit of measure conversion, exclusion of values generated from mixtures). The applied selection procedure reduced the dimension of the OFT-DB from initial 2695 ecotoxicity data to 1956 individual data (of which 1058 chronic tests and 898 acute tests) (Saouter et al. 2018). PPDB contains ecotoxicity data for 1562 substances. PPDB provides no detail on experimental conditions; thus, the selection procedure only aimed to prioritise chronic results.
2.4.2 Calculation of the effect factors (EfFs)
In USEtox® 2.1, EfFs for freshwater toxicity were derived from species sensitivity distribution curves (SDD) (Fantke et al. 2017; Posthuma et al. 2019), whereas for human toxicity were derived from the best estimate (i.e. best available data) of effect concentrations (McKone et al. 2006; Jolliet et al. 2006; Rosenbaum et al. 2008; Fantke et al. 2017).
In June 2018, the UNEP-SETAC LCI brought together approximately 40 experts from all over the world (including one of the authors, as representative of EC-JRC) to a 5-day workshop to agree on new recommendations on life cycle impact assessment models (UNEP 2019). Concerning the freshwater toxicity impact category, agreements pointed to underpin effect modelling on a concentration domain of the species sensitivity distribution (SSD) curve that is close to the domain of environmental (ambient) concentrations. The potentially affected fraction of species (PAF) should be based on HC20 (hazard concentration affecting 20% of the species) identified on an SSD curve of chronic EC10 (concentration producing a biological effect on 10% of the tested population), i.e. an ecosystem-representative dose–response curve built selecting the effect concentration generating a chronic effect response in 10% of the test population for all the species considered. Figure 1 illustrates the connection between HC20 and EC10: endpoints derived from dose–response curves (one per species—Daphnia Magna as example in graph on top-right corner) are interpolated to draw a sigmoidal SSD curve representative for the entire ecosystem. The original USEtox® method was instead based on HC50 derived from SSD built with chronic EC50. This new approach allows the generation of substance-level toxicity values that are based on chronic effect making use of the large amount of available EC10 chronic reference points (being chronic EC50 data very scarce). The final values derived with this new approach resulted more in line with official toxicity rankings of substances in EU environmental risk assessment as well as classification and labelling of products as demonstrated in Saouter et al. (2019b).
Starting from the new ecotoxicity database described in section 2.4.1, the following operations were performed to derive final HC20 values based on chronic EC10 SSD curves (Saouter et al. 2018):
-
All toxicity data were extrapolated to chronic EC10 equivalents using the following extrapolation factors: 0.1 for acute EC50 and 0.3 for chronic EC50 (Posthuma et al. 2019). Chronic toxicity data were always prioritised over acute when available for the same species.
-
When toxicity data for at least two species were available, the HC20 was directly derived from the SSD curve (chronic EC10). However, the fewer data the lower the reliability. In fact, uncertainty is estimated to be of 4 orders of magnitude when only two species are available (Van Zelm et al. 2007).
When toxicity data were available for only one species, no SSD curve can be derived. The only data available are considered to be equivalent to an HC50, starting hypothesis in USEtox® documentation. Extrapolation factors of 0.41, 0.34 and 0.53 were proposed to estimate HC20 for organic, inorganic (including metals and organometallic) chemicals, and for chemicals classified in ECHA as petroleum products respectively. Extrapolation factors proposed above were calculated as ratio between HC20 and HC50, derived from SSD curves, built using ecotoxicological data from REACH-DB for at least three species.
-
The freshwater ecotoxicity EfFs were calculated as Eq. (3), where 0.2 represents the fraction of species potentially disappearing (20%). This allows having an effect factor which is higher for chemicals which are more toxic (and then have an HC which is lower).
$$\mathrm{EfF=0.2/HC_{20}}$$(3)
2.4.3 Quality assessment of ecotoxicological data
For the majority of substances, HC20 was derived using toxicity data for less than four species (Saouter et al. 2019a, b). Therefore, a quality scoring system was developed to estimate the reliability of the SSD curve and, consequently, of the derived HC20 and EfF values. This quality score for ecotoxicity data (QSe) was based on (i) the number of available toxicity data on different species, (ii) number of available toxicity data covering different taxonomic groups (e.g. algae, crustaceans and fish) and (iii) the number of toxicity data extrapolated from acute EC50 or chronic EC50 to chronic EC10 Eq. (4).
The logarithmic function was selected as it allows reducing the relevance of large numbers (e.g. lots of species available or taxonomic groups) on the QSe. Three taxonomic groups (algae, crustaceans and fish) and five different species are deemed necessary to improve ecological realism (Owsianiak et al. 2019). Once the criteria of having data on three taxonomic group and five different species are met, the extent to which they are satisfied has a lower influence on the QSe. Hence, a higher number of species or taxonomic groups would increase the QSe to a smaller extent.
The contribution of the extrapolation has a lower relevance on the QSe compared to number of species and taxonomic groups (Table 2). The 0.1 exponent was arbitrarily chosen to produce a decreasing curve not strongly penalising results with high number of extrapolations since extrapolated data are anyway considered suitable for SSD curves (Saouter et al. 2018; Owsianiak et al. 2019).
2.4.4 The “Ecotox explorer”: a web interactive tool to explore underlying ECOTOX data
Authors developed an online interactive application to further explore the underlying ecotoxicological data and physico-chemical properties used to derive ECOTOX CFs. The “Ecotox explorer” application is accessible at: https://eplca.jrc.ec.europa.eu/ecotox.html.
It guides users in: (i) understanding underlying data, (ii) comparing characterised chemicals, (iii) supporting the interpretation of results by providing a summary of the key research insights from Saouter et al. (2018) and (iv) finding additional information and external resources. 4599 chemicals are available in the “Ecotox explorer”. Substances either protected by confidentiality agreement in REACH-DB or whose CF was directly taken from USEtox® 2.1 are not displayed in the application.
2.5 Toxicological data and calculation principles to derive human toxicity non-cancer Effect Factors
2.5.1 Human toxicity non-cancer database
Data from REACH-DB, OFT-DB and PPDB were used to calculate human toxicity non-cancer effect factors. REACH-DB contains 28440 and 12941 repeated dose toxicity (RDT) test results for ingestion and inhalation exposure routes, respectively. Reliability of data was assessed by means of quality criteria, such as: (i) Klimisch score (Klimisch et al. 1997), (ii) adequacy of the underpinning study, (iii) GLP compliance and (iv) experiment guidelines. In addition, route of administration and inhalation exposure type were considered exclusively for inhalation exposure. All these variables were also used to assign three quality scores (high, intermediate and low) for human toxicity (QSht). The full procedure and related scores are reported in (Saouter et al. 2018). Regarding human toxicity non-cancer, OFT-DB contains 3337 experimental observations, 97% of which refer to ingestion exposure. In the OFT-DB, there are toxicity data used to define either health-based guidance values (HBGV), such as ADI (acceptable daily intake), AOEL (acceptable operator exposure limit) and ARfD (acute reference dose), or margin of exposure/safety data. When available, data used for the derivation of ADI, AOEL and ARfD were always prioritised over the others. USEtox® and PPDB, which contain acute LD50 (lethal dose for 50% of a group test animals) for mammals for both ingestion (2058 test results) and inhalation (968 test results) exposure routes, were used to complement the other database (REACH-DB and OFT-DB) when data were missing.
For each chemical, among toxicity data satisfying the quality requirements, a further selection was performed based on the endpoint (chronic was preferred, then semi-chronic and acute) and on the reference point (no observed effect levels always preferred over lowest observed effect levels). Last, when more than one value was available, the lowest value was retained, adopting a conservative approach.
2.5.2 Calculation of human non-cancer Effect Factors
Lifetime ED50 (i.e. dose producing an effect on 50% of the tested population) for human via ingestion and inhalation exposures are the starting points to calculate the EfFs for human toxicity non-cancer impact in USEtox®. Lifetime ED50 was calculated using conversion factors for endpoint, reference point and species, according to USEtox® method (Fantke et al. 2017). To enlarge the amount of available data, USEtox® recommends the use of route-to-route (R2R) extrapolation. However, this procedure is suggested only in presence of verified systemic toxicity, which is infrequently stated in databases. Despite this, R2R was applied and the obtained data were divided by an arbitrary factor of 10 to cover uncertainty associated with the extrapolation.
2.6 Derivation of human toxicity cancer effect factors
REACH-DB, OFT-DB and PPDB do not include the data needed to calculate the human toxicity cancer impact as per the USEtox® method. Therefore, the human toxicity cancer effect factors of USEtox® 2.1 were retained. Around new 400 chemicals were added in EF 3.0 with EfF equal to 0 (verified absence of carcinogenic effect). These substances were already characterised in USEtox® but not included in the EF version 2.0.
2.7 Robustness factors
The main features and equations of the USEtox® model allow addressing impacts due to non-dissociating and non-amphiphilic organic substances. Applying the model to assess metals and inorganics results in a greater uncertainty of the characterisation factors. In fact, the robustness factors (RFs) were needed to balance the lower robustness in fate modelling for non-organic compounds in USEtox® (Fantke et al. 2015a). Moreover, during the EF pilot phase, metals were observed to dominate the impact scores of all representative products. To overcome these limitations (i.e. the uncertainty of non-organic substances and the dominance of metals), RFs were applied to each CF, reflecting both the model uncertainties in dealing with non-organic substances and the specificities of certain groups of substances (Saouter et al. 2018) (Table 3).
According to Pellston recommendations regarding essential metals (UNEP 2019), a lower RF was applied to essential metals. This choice takes into account deficiency in essential metals that may affect humans (Aggett et al. 2015; Zoroddu et al. 2019) and ecosystems (Wintz et al. 2002), determining that toxicity might be overestimated. A typical example is the zinc, usually dominating human toxicity results, while deficiency in global population is pretty common (around 17% of population according to Wessells and Brown (2012)). Ideally, as recommended in the Pellston workshop, only the fraction of population that is at risk should be considered (Owsianiak et al. 2019). This aspect requires metals-specific data, which are currently missing. For the time being, until essentiality of metals will be addressed in the USEtox® model, RFs are applied to mitigate the overestimation of impacts.
2.8 Characterisation factors
As mentioned in the previous section, CFs calculated for mono-constituent organic substances should be considered within the domain of applicability of the USEtox® model. However, this model was employed also to derive CFs for multi-constituents, UVCB (unknown or variable composition, complex reaction products or biological materials) and organometallic substances.
USEtox® provides also CFs for 27 cationic metals. Those have been developed via a multi-years stakeholder collaborative effort (Westh et al. 2015) and all CFs for cationic metals were retained as such in EF3.0. Hence, no modification to the USEtox® 2.1 input data for metals was performed, and EfFs for ecotoxicity were based on HC50. However, since emissions of different forms of metals are reported in life cycle inventories, the following interventions were made:
-
All elementary flows in EF3.0 nomenclature corresponding exactly to the oxidative form present in USEtox® were directly associated with the equivalent USEtox® CFs (27 chemicals).
-
USEtox® CFs for metals were assigned also to the non-oxidative form of metals available in EF3.0 nomenclature (23 chemicals). For arsenic, iron and antimony, the average of the CFs of their two oxidative forms available in USEtox® was used. For chromium-related elementary flows, the CFs of chromium (VI) were always associated, adopting a conservative approach, being Cr (VI) more toxic than Cr (III).
It would be advisable to perform in future sensitivity analysis to use HC20 for assessing ecotoxicological potential of metals, in order to assure consistency with calculation principles used for organic and inorganic chemicals.
2.8.1 Characterisation of generic group of substances
In the nomenclature of the EF 2.0 reference package, several elementary flows were reported under a generic name, such as “insecticides unspecified”, “polycyclic aromatic hydrocarbons” (PAHs) and “volatile organic carbons” (VOCs). These groups of substances are not present as such in the REACH-DB, OFT-DB and PPDB databases. To avoid leaving these flows uncharacterised, an approximate CF (proxy) was proposed to characterise them following specific assumptions. The proxy CFs were calculated as the 50th percentile or the weighted average of the CFs of available substances pertaining to each generic group. For instance, CFs for “insecticides, unspecified” were derived as the 50th percentile of all the available CFs for insecticides (180). The list of proxies CFs and the rule for their derivation is available in the supplementary materials (Table SM2).
2.8.2 Assessing characterisation factors uncertainty
The uncertainty of the CFs resulting from the use of new input data and calculation principles was evaluated. The qualitative assessment of the specific uncertainty behind every parameter used in the USEtox® model is provided in Saouter et al. (2018). In addition, geometric coefficient of variation (for physicochemical properties) and standard deviation (for ecotoxicological data) were provided in Saouter et al. (2018) when possible.
As stated by USEtox® developers ‘contributions of 1%, 5% or 90% to the total toxicity score can be interpreted as essentially equal, but significantly larger than those of a chemical contributing to less than one per thousand or less than one per million of the total score. […] This means that for LCA practitioners, these toxicity factors are very useful to identify the ten or twenty most important toxics pertinent for their applications” (Rosenbaum et al. 2008, p. 554) and “It should be stressed that the characterization factors are useful for a first tier assessment’ (Fantke et al. 2015b, p.6).
Furthermore, authors suggest that ‘in case a substance appears to dominantly contribute to the impact scores for toxicity, it is recommended to verify the reliability of the chemical-specific input data for this substance and to improve the data whenever possible’ (Fantke et al. 2015b, p. 6).
These recommendations are equally valid for the work presented in this study.
3 Results and discussion
According to the method illustrated in the previous sections, results of the derivation procedures for physico-chemical properties (used to calculate FFs and XFs), EfFs and CFs for toxicity-related impact categories are reported hereafter. Moreover, EC-JRC adaptions to the original USEtox® 2.1 model are summarised.
3.1 Adaptation of USEtox® model for EF 3.0
For deriving EF 3.0 characterisation factors, USEtox® model was adapted to account for the new data sources, the new EfF calculation principles and the chemical type’s uncertainty. All these modifications are synthesised in Fig. 2. It can be read as an illustrated table: each column reports a variable (data source, FF, XF, EfF and RF) and each row the USEtox® model versions (1.0 as adopted in EF2.0, 2.1, and its adaptation for EF 3.0). The colour of the frames of each box brings information on the origin: all information in boxes with the same colour were introduced in the same version of the model. For instance, the effect factor for human toxicities in EF 3.0 is reported in a blue box (colour associated to USEtox® 1.0), meaning that its derivation principle applied in USEtox® 1.0 was maintained in EF 3.0; instead, fate and exposure factors in EF 3.0 follow the calculation principle introduced in USEtox® 2.1 (yellow frame). New sources of toxicological data and ecotoxicity effect calculation principle were introduced in EF 3.0 (green frame).
3.1.1 Derived physico-chemical properties
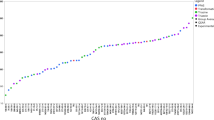
Physico-chemical properties were derived as explained in section 2.3 for a set of 10270 chemicals and adopted to calculate FFs and ExFs with the USEtox® 2.1 model. Figure 3 reports the number of final data obtained from the different sources and their QSpc for the six physicochemical properties available in the data sources used in this study. QSpc for degradation rates in soil and sediments is the same assigned to the degradation rate in water.
Quality scores for physico-chemical properties data for 10,270 chemicals. QSpc are reported for each of the six physicochemical properties available in the new data sources: degradation rate in water (kDegW), Henry’s law constant (KH25C), organic carbon/water partition (Koc), n-octanol/water partition (Kow), vapour pressure (Pvap25) and water solubility (Sol25). Bars report quality score according to Table 1, from highest (darker colour) to lowest (lighter colour)
The priority order (described in section 2.3.3) favours experimental and highly trustworthy data and adopts model predictions only when no other data are available. As a consequence, Fig. 3 shows a very limited number of values labelled as ‘Low reliability (REACH-DB)’ and ‘EPISuite estimation, out AD’, being model estimations associated to a lower level of reliability. The number of data retrieved from REACH-DB varies substantially among the physicochemical properties: degradation rate in water is the most abundant property in REACH-DB, whereas Henry law constant is the less reported information. Data gap filling strategies allowed to retrieve values for around half of the chemicals for each parameter. When all other options were not available, physicochemical properties were collected from USEtox® 2.1 input dataset (1754 chemicals).
3.1.2 Derived effect factors
Ecotoxicological EfFs were derived for 6764 chemicals. Table 4 reports the number of chemicals by EfF quality score. The large majority of chemicals has a low-quality score due to the lack of toxicity data needed for the calculation. In fact, toxicological data for only one species were available for almost 2000 chemicals.
Human toxicity non cancer EfFs were retrieved for 5071 chemicals. Table 5 reports the number of chemicals by quality score for both ingestion and inhalation exposure routes. Extrapolation was higher in the ingestion-to-inhalation route than in the ingestion route. Although OFD-DB was the preferred source, most of the chemicals are classified under REACH-DB categories as this database contains the largest amount of data and it was prioritised compared to PPDB and USEtox® 2.1.
3.1.3 Derived characterisation factors
USEtox® 2.1 provides data and CFs for 3077 organic chemicals and for 27 cationic metals. The new set of CFs generated by EC-JRC for the EF3.0 consists of 6708 chemicals, including mainly organic substances but also inorganics, metals, organometallic, petroleum products, UVCB and groups for which proxy CF values are provided.
CFs were calculated for 6038, 1039 and 3317 different chemicals for ECOTOX, HTOX_c and HTOX_nc impact categories, respectively. Table 6 illustrates the difference between the number of characterised chemicals using USEtox® in EF 3.0 and EF 2.0 (proxies excluded), as well as the percentage variation between them. ECOTOX shows the highest number of characterised chemicals. However, HTOX_nc reports by far the major increase in comparison with EF 2.0 (+437%).
Regarding data sources, REACH-DB is by far the primary source of data for ECOTOX and HTOX_nc, for both physicochemical (XFs and FFs) and toxicological (EFs) data (Table 7). FF and XF are calculated by means of several physico-chemical properties which may be retrieved from different sources. In Table 7, “USEtox® 2.1” indicates the number of chemicals for which all physico-chemical were directly taken from USEtox® model, whereas “REACH-DB” indicates substances for which new fate and exposure factors were calculated. For the latter, data were retrieved primarily from REACH-DB or by applying data-gap filling procedure. EfFs for several factors were retrieved from USEtox®: it still significantly contributes to the overall number of characterised chemicals. In many occurrences, its CFs were updated using more reliable FFs and XFs retrieved from the REACH-DB for several chemicals. For instance, despite no new EfFs were calculated for HTOX_c, 410 CFs were updated with REACH-DB physicochemical properties. The total reported in Table 7 refers to individual substances.
The ratio between CFs for each toxicity-related impact categories in EF3.0 and the original values in USEtox® 2.1 was evaluated to explore the effect of adding new data sources and derivation procedures (Fig. 4). Similarly, ratios for EfFs, FFs and XF (iF for human toxicity impact categories) were added to understand which of the underlying factors were causing the observed difference. Only chemicals of EF 3.0 in common with the USEtox® 2.1 database and not taken as such from it were analysed. The number of chemicals selected is reported in brackets next to each impact category. This comparison was performed at midpoint level, choosing “emissions to freshwater” for ECOTOX whereas “emissions to urban air” for HTOX_c and HTOX_nc. All CFs with less than one order of magnitude of difference of the ratio are lying within the range identified in the graph by the two dashed grey horizontal lines.
The greatest difference is observed for the freshwater ecotoxicity impact category, where EF 3.0 values tend to be higher than the original USEtox® CFs. This reflects the more conservative approach applied to derive the effect factor in EF 3.0. In fact, the majority of the variability of the CFs is explained by the variability of the EfFs that report a significant variation in line with the values of the ratio between CFs (Fig. 4). 65% of ECOTOX CFs in EF 3.0 are within one order of magnitude of difference with their equivalent in USEtox® 2.1 and 91% within two orders of magnitude.
The highest difference for the freshwater ecotoxicity impact category is registered for "dodecylbenzenesulphonic acid, calcium salt". Comparing EF3.0 and USEtox® factors (i.e. EfF, XF and FF) fro this susbstance, it is observed that the exposure factor is the main driver of the difference, despite the EfF is generally the main driver for disalignment between EF3.0 and USEtox CFs. In fact, the ratio between XFs shows a difference of six orders of magnitude, whereas the ratio between EfFs and FFs is close to 1. The XF depends on physico-chemical properties which were estimated from EPISuite QSAR models in USEtox® (Fantke et al. 2017). On the contrary, all physico-chemical properties for “dodecylbenzenesulphonic acid, calcium salt” in EF3.0 are labelled as “key values used for safety assessment” in REACH database. In light of this, the EF3.0 CF may be considered more reliable being derived using more trustworthy input data as well as ensuring traceability of the input data.
HTOX_c CFs are very similar between EF 3.0 and USEtox® 2.1, the only differences are specular to variation in iFs, as EfFs were not modified. HTOX_nc CFs in EF 3.0 are quite similar to their equivalent in USEtox® 2.1, in fact, 80% are within one order of magnitude of difference and 89% within two orders of magnitude. Moreover, regarding the different exposure routes, EF3.0 EfFs are more similar with USEtox®’s ones for the inhalation exposure as the interquartile range is shorter.
Despite the new data sources, 317 chemicals listed in the EF 3.0 package remain uncharacterised in all toxicity-related impact categories because no physicochemical or toxicity data could be found in the consulted databases. This lack of data can be due to different reasons:
-
1.
Incomplete or unreliable data in the databases;
-
2.
Substance that benefits from reduced information requirements according to REACH regulation. These substances have tonnage between 1 and 10 tons per year and they are predicted to have low risk;
-
3.
Substances not relevant for toxicity-related impact categories (e.g. helium).
The supplementary material (Table SM3) reports the substances for which the difference between the CFs of EF.3.0 and USEtox is higher than 3 orders of magnitude (26 for ECOTOX and 5 for HTOX_nc)
4 Conclusions
The new set of CFs for toxicity-related impact categories derived by EC-JRC for the EF 3.0 contains CFs for 6038, 1024 and 3317 chemicals for the freshwater ecotoxicity, human toxicity cancer and human toxicity non-cancer impact categories, respectively. A strategy based on existing CFs allowed to characterise also generic groups of substances, such as PAHs. The larger number of characterised chemicals in EF 3.0 ensures a broader coverage of Life Cycle Inventories that brings new insights on elementary flows previously uncharacterised in EF 2.0.
The new set of CFs was developed to overcome USEtox® limitations that arose during the EF pilot phase. Trusted multiple data sources (ECHA, EFSA, PPDB and USEtox®) were consulted to build this enlarged dataset and to ensure a satisfactory level of reliability of the input data. CFs were calculated for all chemicals available, including non-organic substances. Robustness factors (RFs) were applied in order to cover the uncertainty of the USEtox® model in dealing with non-organic compounds. The adoption of HC20 for the calculation of ECOTOX EfFs produces more conservative CFs that align toxicity data for LCIA and risk assessment. The “Ecotox explorer” interactive web application is a supportive tool in understanding the derivation of ECOTOX CFs and their underlying data. It is available at: https://eplca.jrc.ec.europa.eu/ecotox.html. Lastly, quality evaluation for each parameter (both toxicological and physicochemical) is provided to support EF 3.0 users in the verification of the reliability of input data, as suggested by USEtox®developers for dealing properly with model uncertainty.
Guidelines for the interpretation of CFs of the EF 3.0 method will be the object of dedicated publications.
While significant efforts have been put in improving the calculations of characterisation factors, in the traceability of data source and in the transparency on data quality, further scientific developments are needed, e.g. to cover more environmental compartments and more substances, to improve the characterisation of metals and inorganics and to ensure consistency in the use of HC for organic and metals.
Data availability
The data underpinning this study are available at these links: characterisation factors at https://eplca.jrc.ec.europa.eu/LCDN/developerEF.xhtml;jsessionid=F2525D4BE85CAE36FCAA7262E8A9C189, underpinning ecotox data at https://eplca.jrc.ec.europa.eu/ecotoxProject.html.
Notes
The Life Cycle Initiative refers to a public–private, multi-stakeholder partnership hosted by the United Nations Environment (UNEP) in collaboration with the Society of Environmental Toxicology and Chemistry (SETAC).
References
Aggett P, Nordberg GF, Nordberg M (2015) Essential metals : assessing risks from deficiency and toxicity. In: Handbook on the toxicology of metals : Volume I: General considerations (4th ed., pp. 281–297). https://doi.org/10.1016/B978-0-444-59453-2.00014-7
Bare J (2011) TRACI 2.0: the tool for the reduction and assessment of chemical and other environmental impacts 2.0. Clean Technol Environ Policy 13:687–696. https://doi.org/10.1007/s10098-010-0338-9
Bassan A, Ceriani L, Richardson J, Livaniou A, Ciacci A, Baldin R, Kovarich S, Fioravanzo E, Pavan M, Gibin D et al (2018) OpenFoodTox: EFSA’s chemical hazards database. https://zenodo.org/record/1252752#.YhOR1zFBxaR
Dorne JL, Richardson J, Kass G, Georgiadis N, Monguidi M, Pasinato L Cappe S, Verhagen H, Robinson T (2017) Editorial: OpenFoodTox: EFSA's open source toxicological database on chemical hazards in food and feed EFSA J 15(1):e15011 3. https://doi.org/10.2903/j.efsa.2017.e15011
EC (2021) 9332 final Commission Recommendation of 16.12.2021 on the use of the Environmental Footprint methods to measure and communicate the life cycle environmental performance of products and organisations
EC (European Commission) (2002) Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off J Eur Union 31(1):24
EC (European Commission) (2006) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off J Eur Union L 369(1):15
EC (European Commission) (2013a) Communication from the Commission to the European Parliament and the Council. Building the Single Market for Green Products Facilitating better information on the environmental performance of products and organisations. COM 196.
EC (European Commission) (2013b) Commission Recommendation (2013/179/EU) of 9 April 2013 on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations Off J Eur Communities L124:(1)216
EC (European Commission) (2020) Communication from the Commission to the European Parliament, the Council, the European economic and social committee and the committee of the regions. Chemicals Strategy for Sustainability Towards a Toxic-Free Environment COM:667
EC (European Commission) (2021a) Initiative on substantiating green claims. Available at https://ec.europa.eu/environment/eussd/smgp/initiative_on_green_claims.htm
EC (European Commission) (2021b) Zero pollution action plan. Available at: https://ec.europa.eu/environment/strategy/zero-pollution-action-plan_en Accessed Mar 2021b
EC-JRC (European Commission - Joint Research Centre) (2003) Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Part II EUR 20418 EN/2
EC-JRC (European Commission - Joint Research Centre) (2018) Environmental Footprint reference package 3.0 (EF 3.0). Available at: https://eplca.jrc.ec.europa.eu/LCDN/developerEF.xhtml (Accessed 2020 Dec)
Fantke P, Bijster M, Hauschild MZ, Huijbregts MAJ, Jolliet O, Kounina A, Magaud V, Margni M, McKone TE, Rosenbaum RK et al (2017) USEtox 2.0 Documentation (Version 1.00). Lyngby, Denmark. ISBN: 978–87–998335–0–4. https://doi.org/10.11581/DTU:00000011.
Fantke P, Huijbregts M, Hauschild M, Margni M, Jolliet O, McKone TE, van de Meent D, Rosenbaum RK (2015a) USEtox® 2.0 Manual: Inorganic Substances (Version 2), available at https://usetox.org/sites/default/files/support-tutorials/USEtox_Manual_inorganics_0.pdf
Fantke P, Huijbregts MAJ, Margni M, Hauschild MZ, Jolliet O, McKone TE, Rosenbaum RK, van de Meent D (2015b) USEtox® 2.0 User Manual (Version 2). http://usetox.org
Fazio S, Biganzoli F, De Laurentiis V, Zampori L, Sala S, Diaconu E (2018) Supporting information to the characterisation factors of recommended EF Life Cycle Impact Assessment methods, version 2, from ILCD to EF 3.0, EUR 29600 EN, European Commission, Ispra ISBN 978–92–79–98584–3. https://doi.org/10.2760/002447
Hauschild MZ, Huijbregts MAJ, Jolliet O, Macleod M, Margni M, van de Meent D, Rosenbaum RK, McKone TE (2008) Building a Model Based on Scientific Consensus for Life Cycle Impact Assessment of Chemicals: The Search for Harmony and Parsimony. Environ Sci Technol 42:7032–7037. https://doi.org/10.1021/es703145t
Hauschild MZ, Potting J (2005) Spatial Differentiation in Life Cycle Impact Assessment - The EDIP2003 methodology. Environ News 80:1–195
Huijbregts MAJ, Guinee JB, Reijnders L (2001) Priority assessment of toxic substances in life cycle assessment. III: Export of potential impact over time and space. Chemosphere 44:59–65. ISSN 0045–6535. https://doi.org/10.1016/S0045-6535(00)00349-0.
ISO (International Standard Organisation) (2006a) ISO 14040. Environmental management -- Life cycle assessment -- Principles and framework
ISO (International Standard Organisation) (2006b) ISO 14044. Environmental management -- Life cycle assessment -- Requirements and guidelines
Jolliet O, Margni M, Charles R, Humbert S, Payet J, Rebitzer G, Rosenbaum RK (2003) IMPACT 2002+: A new life cycle impact assessment methodology. Int J Life Cycle Assess 8:324–330. https://doi.org/10.1007/BF02978505
Jolliet O, Rosenbaum RK, McKone TE, Scheringer M, van Straalen N, Wania F (2006) Establishing a Framework for Life Cycle Toxicity Assessment. Findings of the Lausanne Review Workshop. Int J Life Cycle Assess 11:209–212
Kemna R, van Elburg M, Li W, van Holsteijn R (2005) MEEuP Methodology, final. Delft, Netherlands
Klimisch HJ, Andreae M, Tillmann U (1997) A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regul Toxicol Pharmacol 25:1–5. https://doi.org/10.1006/rtph.1996.1076 (PMID: 9056496)
Lewis KA, Tzilivakis J, Warner DJ, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess an International Journal 22(4):1050–1064. https://doi.org/10.1080/10807039.2015.1133242
McKone TE, Enoch KG (2002) CalTOX, A multimedia total exposure model spreadsheet user’s guide. Version 4.0. Lawrence Berkeley Natl Lab. Available at https://dtsc.ca.gov/caltox/
McKone TE, Kyle AD, Jolliet O, Olsen SI, Hauschild MZ (2006) Dose-Response Modeling for Life Cycle Impact Assessment - Findings of the Portland Review Workshop. Int J Life Cycle Asses 11:137–140
Owsianiak M, Fantke P, Posthuma L, Saouter E, Vijver MG, Backhaus T, Schlekat T, Hauschild MZ (2019) Global Guidance for Life Cycle Impact Assessment Indicators Volume 2. Chapter 7 Ecotoxicity. www.lifecycleinitiative.org/training-resources/global-guidance-for-life-cycle-impact-assessment-indicators-volume-2/
Posthuma L, van Gils J, Zijp MC, van de Meent D, de Zwart D (2019) Species Sensitivity Distributions for Use in Environmental Protection, Assessment and Management of Aquatic Ecosystems for 12,386 Chemicals. Environ Toxicol Chem 38:905–917. https://doi.org/10.1002/etc.4373
PPDB (Pesticides Properties DataBase) (2017) The University of Hertfordshire Agricultural Substances Database Background and Support Information. Available at http://sitem.herts.ac.uk/aeru/ppdb/
Rosenbaum RK, Bachmann TM, Gold LS, Huijbregts MAJ, Jolliet O, Juraske R, Koehler A, Larsen HF, MacLeod M, Margni M et al (2008) USEtox—the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 13:532–546. https://doi.org/10.1007/s11367-008-0038-4
RStudioTeam (2016) RStudio: Integrated Development Environment for R. http://www.rstudio.com/
Sala S, Reale F, Cristobal Garcia J, Marelli L, Pant R (2016) Life cycle assessment for the impact assessment of policies. EUR 28380 EN. https://doi.org/10.2788/318544
Saouter E, Aschberger K, Fantke P, Hauschild MZ, Bopp SK, Kienzler A, Paini A, Pant R, Secchi M, Sala S (2017a) Improving substance information in USEtox®, part 1: Discussion on data and approaches for estimating freshwater ecotoxicity effect factors. Environ Toxicol Chem 36:3450–3462. https://doi.org/10.1002/etc.3889
Saouter E, Aschberger K, Fantke P, Hauschild MZ, Kienzler A, Paini A, Pant R, Radovnikovic A, Secchi M, Sala S (2017b) Improving substance information in USEtox®, part 2: Data for estimating fate and ecosystem exposure factors. Environ Toxicol Chem 36:3463–3470. https://doi.org/10.1002/etc.3903
Saouter E, Biganzoli F, Ceriani L, Versteeg D, Crenna E, Zampori L, Sala S, Pant R (2018) Environmental Footprint : Update of Life Cycle Impact Assessment Methods – Ecotoxicity freshwater, human toxicity cancer, and non- cancer. JRC technical report. EUR 29495 EN, Publications Office of the European Union Luxembourg ISBN 978–92–79–98182–1. https://doi.org/10.2760/178544.
Saouter E, Biganzoli F, Pant R, Sala S, Versteeg D (2019a) Using REACH for the EU Environmental Footprint: Building a Usable Ecotoxicity Database, Part I. Integr Environ Assess Manag 15:783–795. Available from: https://doi.org/10.1002/ieam.4168
Saouter E, Wolff D, Biganzoli F, Versteeg D (2019b) Comparing Options for Deriving Chemical Ecotoxicity Hazard Values for the European Union Environmental Footprint. Part II Integr Environ Assess Manag 15(5):796–807. https://doi.org/10.1002/ieam.4169
Simulations Plus (2016) ADMET PredictorTM. https://www.simulations-plus.com/software/admetpredictor
S‐IN Soluzioni Informatiche (2015) Further development and update of EFSA's Chemical Hazards Database. EFSA Supporting Publication 12(7):EN‐823-884. https://doi.org/10.2903/sp.efsa.2015.EN‐823
UNEP (United Nations Environment Program) (2019) Global Guidance for Life Cycle Impact Assessment Indicators Volume 2. https://www.lifecycleinitiative.org/training-resources/global-guidance-for-life-cycle-impact-assessment-indicators-volume-2/
US-EPA (United States Environmental Protection Agency) (2012) BIOWINTM User’s Guide (v4.10). Available at: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface
US-EPA (United States Environmental Protection Agency) (2018) Estimation Programs Interface SuiteTM for Microsoft® Windows, v 4.11. United States Environmental Protection Agency, Washington, DC, USA
USEtox (2018) Official USEtox Download website. https://usetox.org/ Accessed 2018 Nov
van Zelm R, Huijbregts MAJ, Harbers JV, Wintersen A, Struijs J, Posthuma L, Van de Meent D (2007) Uncertainty in msPAF-Based Ecotoxicological Effect Factorsfor Freshwater Ecosystems in Life Cycle Impact Assessment. Integr Environ Assess Manag 3:203–210. https://doi.org/10.1897/ieam_2006-013.1 (PMID: 17477288)
van Zelm R, Huijbregts MAJ, van de Meent D (2009) USES-LCA 2.0—a global nested multi-media fate, exposure, and effects model. Int J Life Cycle Assess 14:282–284. https://doi.org/10.1007/s11367-009-0066-8
Wegmann F, Cavin L, Macleod M, Scheringer M, Hungerbu K (2009) The OECD software tool for screening chemicals for persistence and long-range transport potential. Environ Model Softw 24:228–237. https://doi.org/10.1016/j.envsoft.2008.06.014
Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS One 7(11):e50568. https://doi.org/10.1371/journal.pone.0050568 (PMID: 23209782)
Westh TB, Hauschild MZ, Birkved M, Jørgensen MS, Rosenbaum RK, Fantke P (2015) The USEtox story: a survey of model developer visions and user requirements. Int J Life Cycle Assess 20:299–310. https://doi.org/10.1007/s11367-014-0829-8
Wintz H, Fox T, Vulpe C (2002) Responses of plants to iron, zinc and copper deficiencies. Biochem Soc Trans 30(4):766–768. https://doi.org/10.1042/bst0300766 (PMID: 12196190)
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129. https://doi.org/10.1016/j.jinorgbio.2019.03.013 (PMID: 30939379)
Acknowledgements
The present study has been financially supported by the Directorate General for the Environment (DG ENV) of the European Commission in the context of the Administrative Arrangement "Application of the consumption footprint indicators in policy analysis” (No 070201/2018/790087/AA/ENV.B.1) and “Technical support for the Environmental Footprint and the Life Cycle Data Network” (EF4) (N °070201/2019/811467/AA/ENV.B.1). The authors want to thank Fulvio Ardente, Sara Corrado and Andrea Amadei for their comments to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Z. Hauschild.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
11367_2022_2033_MOESM1_ESM.docx
Supplementary file1 (DOCX 34 KB) SM1. Criteria used for classifying quality scores for physico-chemical properties from REACH-DB. Taken from (Saouter et al. 2018). SM2. List of proxies and the rule for their derivation. SM3. Substances for which the differences between EF3.0 and USEtox CF is more than 3 orders of magnitude.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sala, S., Biganzoli, F., Mengual, E.S. et al. Toxicity impacts in the environmental footprint method: calculation principles. Int J Life Cycle Assess 27, 587–602 (2022). https://doi.org/10.1007/s11367-022-02033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-022-02033-0