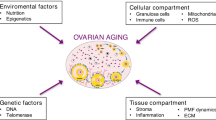
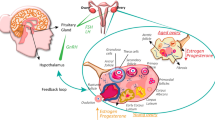
Abstract
Ovarian reserve is a term used to estimate the total number of immature follicles present in the ovaries. Between birth and menopause, there is a progressive decrease in the number of ovarian follicles. Ovarian aging is a continuous physiological phenomenon, with menopause being the clinical mark of the end of ovarian function. Genetics, measured as family history for age at the onset of menopause, is the main determinant. However, physical activity, diet, and lifestyle are important factors that can influence the age of menopause. The low estrogen levels after natural or premature menopause increased the risk for several diseases, resulting in increased mortality risk. Besides that, the decreasing ovarian reserve is associated to reduced fertility. In women with infertility undergoing in vitro fertilization, reduced markers of ovarian reserve, including antral follicular count and anti-Mullerian hormone, are the main indicators of reduced chances of becoming pregnant. Therefore, it becomes clear that the ovarian reserve has a central role in women’s life, affecting fertility early in life and overall health later in life. Based on this, the ideal strategy for delaying ovarian aging should have the following characteristics: (1) be initiated in the presence of good ovarian reserve; (2) maintained for a long period; (3) have an action on the dynamics of primordial follicles, controlling the rate of activation and atresia; and (4) safe use in pre-conception, pregnancy, and lactation. In this review, we therefore discuss some of these strategies and its feasibility for preventing a decline in the ovarian reserve.

Similar content being viewed by others
References
Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann N Y Acad Sci. 2008;1127:1–9.
Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91.
Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–33.
Crawford NM, Steiner AZ. Age-related infertility. Obstet Gynecol Clin North Am. 2015;42:15–25.
Overland MR, Li Y, Derpinghaus A, Aksel S, Cao M, Ladwig N, Cunha GR, Himelreich-Perić M, Baskin LS. Development of the human ovary: fetal through pubertal ovarian morphology, folliculogenesis and expression of cellular differentiation markers. Differentiation. 2023;129:37–59.
Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update. 2022;28:172–89.
Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162:R19–33.
The 2022 Hormone Therapy Position Statement of The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;(29):767–94.
Katainen R, Engblom JR, Polo-Kantola P. Climacteric-related symptoms in menopause transition and beyond: a prospective 19-year follow-up study on previously hysterectomized women. Menopause. 2018;25:890–6.
McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 2008;61:4–16.
Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–40.
El Khoudary SR, Greendale G, Crawford SL, Avis NE, Brooks MM, Thurston RC, Karvonen-Gutierrez C, Waetjen LE, Matthews K. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause. 2019;26:1213–27.
Ceylan B, Özerdoğan N. Factors affecting age of onset of menopause and determination of quality of life in menopause. Turk J Obstet Gynecol. 2015;12:43–9.
Newson L. Menopause and cardiovascular disease. Post Reprod Health. 2018;24:44–9.
de Kat AC, Broekmans FJM, Lambalk CB. Role of AMH in prediction of menopause. Front Endocrinol (Lausanne). 2021;12:733731.
Depmann M, Broer SL, van der Schouw YT, Tehrani FR, Eijkemans MJ, Mol BW, Broekmans FJ. Can we predict age at natural menopause using ovarian reserve tests or mother’s age at menopause? Syst Lit Rev Menopause. 2016;23:224–32.
Robertson DM. Inhibins and activins in blood: predictors of female reproductive health? Mol Cell Endocrinol. 2012;359:78–84.
Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105:3361–73.
Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab. 2011;25:861–73.
Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ. Testing ovarian reserve to predict age at menopause. Maturitas. 2009;63:280–91.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6.
Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol. 2021;9:672890.
Podfigurna-Stopa A, Czyzyk A, Grymowicz M, Smolarczyk R, Katulski K, Czajkowski K, Meczekalski B. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest. 2016;39:983–90.
Malek AM, Vladutiu CJ, Meyer ML, Cushman M, Newman R, Lisabeth LD, Kleindorfer D, Lakkur S, Howard VJ. The association of age at menopause and all-cause and cause-specific mortality by race, postmenopausal hormone use, and smoking status. Prev Med Rep. 2019;15:100955.
Blümel JE, Mezones-Holguín E, Chedraui P, Soto-Becerra P, Arteaga E, Vallejo MS. Is premature ovarian insufficiency associated with mortality? A three-decade follow-up cohort. Maturitas. 2022;163:82–7.
Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52:303–7.
Popat VB, Calis KA, Vanderhoof VH, Cizza G, Reynolds JC, Sebring N, Troendle JF, Nelson LM. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab. 2009;94:2277–83.
Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, Furmaniak J, Smith BR, Merino MJ, Nelson LM. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46, XX spontaneous premature ovarian failure. Fertil Steril. 2005;84:958–65.
Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9.
Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, Rubinow DR, Nelson LM. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J Clin Endocrinol Metab. 2011;96:E278–87.
Bellver J, Rodríguez-Tabernero L, Robles A, Muñoz E, Martínez F, Landeras J, García-Velasco J, Fontes J, Álvarez M, Álvarez C, Acevedo B, Group of interest in Reproductive Endocrinology (GIER) of the Spanish Fertility Society (SEF). Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet. 2018;35:25–39.
Ehrlich S. Effect of fertility and infertility on longevity. Fertil Steril. 2015;103:1129–35.
Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, De Placido G, Alviggi C. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med. 2019;61:24–9.
Drakopoulos P, Bardhi E, Boudry L, Vaiarelli A, Makrigiannakis A, Esteves SC, Tournaye H, Blockeel C. Update on the management of poor ovarian response in IVF: the shift from Bologna criteria to the Poseidon concept. Ther Adv Reprod Health. 2020;14:2633494120941480.
Abu-Musa A, Haahr T, Humaidan P. Novel physiology and definition of poor ovarian response; clinical recommendations. Int J Mol Sci. 2020;21:2110.
Zhuang J, Li H, Li X, Tian D, Yang D, Zhu M. The incidence of unexpected poor ovarian response in Chinese young women. Medicine (Baltimore). 2019;98:e14379.
Venetis CA, Kolibianakis EM, Tarlatzi TB, Tarlatzis BC. Evidence-based management of poor ovarian response. Ann N Y Acad Sci. 2010;1205:199–206.
Badawy A, Wageah A, El Gharib M, Osman EE. Prediction and diagnosis of poor ovarian response: the dilemma. J Reprod Infertil. 2011;12:241–8.
Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18:1137–9.
Zhang Y, Zhang C, Shu J, Guo J, Chang HM, Leung PCK, Sheng JZ, Huang H. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. 2020;26:247–63.
Wang Z, Yang A, Bao H, Wang A, Deng X, Xue D, Tan H, Zhou Y, Wu C, Chen ZJ, Shi Y. Effect of dehydroepiandrosterone administration before in vitro fertilization on the live birth rate in poor ovarian responders according to the Bologna criteria: a randomised controlled trial. BJOG. 2022;129:1030–8.
Hart RJ. Stimulation for low responder patients: adjuvants during stimulation. Fertil Steril. 2022;117:669–74.
Muharam R, Sumapraja K, Pratama G, Azyati M, Prabowo KA. Impact of IVF on the timing and symptoms of menopause. Int J Womens Health. 2021;13:889–93.
Bai L, Pan H, Zhao Y, Chen Q, Xiang Y, Yang X, Zhu Y. The exploration of poor ovarian response-related risk factors: a potential role of growth differentiation factor 8 in predicting ovarian response in IVF-ET patient. Front Endocrinol (Lausanne). 2021;12:708089.
Whang J, Ahn C, Kim S, Seok E, Yang Y, Han G, Jo H, Yang H. Effects of repeated ovarian stimulation on ovarian function and aging in mice. Dev Reprod. 2021;25:213–23.
Elder K, Mathews T, Kutner E, Kim E, Espenberg D, Faddy M, Gosden R. Impact of gonadotrophin stimulation for assisted reproductive technology on ovarian ageing and menopause. Reprod Biomed Online. 2008;16:611–6.
de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, van Leeuwen FE, OMEGA-project group. Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod. 2003;18:1544–52.
de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE, OMEGA-project group. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77:978–85.
Szmidt NA, Bhattacharya S, Maheshwari A. Does poor ovarian response to gonadotrophins predict early menopause? A retrospective cohort study with minimum of 10-year follow-up. Hum Fertil (Camb). 2016;19:212–9.
Kasaven LS, Saso S, Getreu N, O’Neill H, Bracewell-Milnes T, Shakir F, Yazbek J, Thum MY, Nicopoullos J, Ben Nagi J, Hardiman P, Diaz-Garcia C, Jones BP. Age-related fertility decline: is there a role for elective ovarian tissue cryopreservation? Hum Reprod. 2022;37:1970–9.
Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction. 2018;156:F11–27.
Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919.
Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022–33.
Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24:1111–20.
Oktay KH, Marin L, Petrikovsky B, Terrani M, Babayev SN. Delaying reproductive aging by ovarian tissue cryopreservation and transplantation: is it prime time? Trends Mol Med. 2021;27:753–61.
Chen J, Han Y, Shi W, Yan X, Shi Y, Yang Y, Gao H, Li Y. Ovarian tissue bank for fertility preservation and anti-menopause hormone replacement. Front Endocrinol (Lausanne). 2022;13:950297.
von Wolff M, Stute P. Cryopreservation and transplantation of ovarian tissue exclusively to postpone menopause: technically possible but endocrinologically doubtful. Reprod Biomed Online. 2015;31:718–21.
Kolibianaki EE, Goulis DG, Kolibianakis EM. Ovarian tissue cryopreservation and transplantation to delay menopause: facts and fiction. Maturitas. 2020;142:64–7.
Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–41.
Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–9.
Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, Henseb JD, Saccon TD, Ocañas SR, Tooley KB, Stout MB, Schneider A, Freeman WM. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech Ageing Dev. 2021;194:111425.
Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17.
Bitto A, Altavilla D, Bonaiuto A, Polito F, Minutoli L, Di Stefano V, Giuliani D, Guarini S, Arcoraci V, Squadrito F. Effects of aglycone genistein in a rat experimental model of postmenopausal metabolic syndrome. J Endocrinol. 2009;200:367–76.
Medina-Contreras J, Villalobos-Molina R, Zarain-Herzberg A, Balderas-Villalobos J. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol Cell Biochem. 2020;475:261–76.
Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda). 2016;31:250–7.
Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, Shulman GI. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021–8.
Schneider AH, Kanashiro A, Dutra SGV, Souza RDN, Veras FP, Cunha FQ, Ulloa L, Mecawi AS, Reis LC, Malvar DDC. Estradiol replacement therapy regulates innate immune response in ovariectomized arthritic mice. Int Immunopharmacol. 2019;72:504–10.
Pollow DP Jr, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1546–52.
Mason JB, Parkinson KC, Habermehl TL. Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J Vis Exp. 2018;132:56638.
Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10:448–56.
Habermehl TL, Underwood KB, Welch KD, Gawrys SP, Parkinson KC, Schneider A, Masternak MM, Mason JB. Aging-associated changes in motor function are ovarian somatic tissue-dependent, but germ cell and estradiol independent in post-reproductive female mice exposed to young ovarian tissue. Geroscience. 2022;44:2157–69.
Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22.
Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20:353–69.
Luo LL, Xu JJ, Fu YC. Rapamycin prolongs female reproductive lifespan. Cell Cycle. 2013;12:3353–4.
Qin X, Du D, Chen Q, Wu M, Wu T, Wen J, Jin Y, Zhang J, Wang S. Metformin prevents murine ovarian aging. Aging (Albany NY). 2019;11:3785–94.
Schneider A, Saccon TD, Garcia DN, Zanini BM, Isola JVV, Hense JD, Alvarado-Rincón JA, Cavalcante MB, Mason JB, Stout MB, Bartke A, Masternak MM. The interconnections between somatic and ovarian aging in murine models. J Gerontol A Biol Sci Med Sci. 2021;76:1579–86.
Isola JVV, Zanini BM, Hense JD, Alvarado-Rincón JA, Garcia DN, Pereira GC, Vieira AD, Oliveira TL, Collares T, Gasperin BG, Stout MB, Schneider A. Mild calorie restriction, but not 17α-estradiol, extends ovarian reserve and fertility in female mice. Exp Gerontol. 2022;159:111669.
Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23:56–73.
Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9.
Flanagan EW, Most J, Mey JT, Redman LM. Calorie restriction and aging in humans. Annu Rev Nutr. 2020;40:105–33.
Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB, Schneider A. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019;41:395–408.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8.
Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015;22:60–7.
Heydari AR, Unnikrishnan A, Lucente LV, Richardson A. Caloric restriction and genomic stability. Nucleic Acids Res. 2007;35:7485–96.
Vermeij WP, Dollé ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, Wu H, Roks AJ, Botter SM, van der Eerden BC, Youssef SA, Kuiper RV, Nagarajah B, van Oostrom CT, Brandt RM, Barnhoorn S, Imholz S, Pennings JL, de Bruin A, Gyenis Á, Pothof J, Vijg J, van Steeg H, Hoeijmakers JH. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537:427–31.
Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–30.
Zhuo Y, Hua L, Feng B, Jiang X, Li J, Jiang D, Huang X, Zhu Y, Li Z, Yan L, Jin C, Che L, Fang Z, Lin Y, Xu S, Li J, Wu D. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. EBioMedicine. 2019;41:623–35.
Henagan TM, Laeger T, Navard AM, Albarado D, Noland RC, Stadler K, Elks CM, Burk D, Morrison CD. Hepatic autophagy contributes to the metabolic response to dietary protein restriction. Metabolism. 2016;65:805–15.
Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ, McManaman JL. High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol Reprod. 2016;94:108.
Nteeba J, Ross JW, Perfield JW 2nd, Keating AF. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reprod Toxicol. 2013;42:68–77.
Scharf A, Pohl F, Egan BM, Kocsisova Z, Kornfeld K. Reproductive aging in Caenorhabditis elegans: from molecules to ecology. Front Cell Dev Biol. 2021;9:718522.
Shi C, Murphy CT. piRNAs regulate a Hedgehog germline-to-soma pro-aging signal. Nat Aging. 2023;3:47–63.
Miller PB, Obrik-Uloho OT, Phan MH, Medrano CL, Renier JS, Thayer JL, Wiessner G, Bloch Qazi MC. The song of the old mother: reproductive senescence in female drosophila. Fly (Austin). 2014;8:127–39.
Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–503.
Steenwinkel TE, Hamre KK, Werner T. The use of non-model Drosophila species to study natural variation in TOR pathway signaling. PLoS ONE. 2022;17:e0270436.
Kulkarni AS, Aleksic S, Berger DM, Sierra F, Kuchel GA, Barzilai N. Geroscience-guided repurposing of FDA-approved drugs to target aging: a proposed process and prioritization. Aging Cell. 2022;21(4):e13596.
Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, Boyle J, Teede HJ. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668–80.
Xu B, Dai W, Liu L, Han H, Zhang J, Du X, Pei X, Fu X. Metformin ameliorates polycystic ovary syndrome in a rat model by decreasing excessive autophagy in ovarian granulosa cells via the PI3K/AKT/mTOR pathway. Endocr J. 2022;69:863–75.
Yao J, Ma Y, Zhou S, Bao T, Mi Y, Zeng W, Li J, Zhang C. Metformin prevents follicular atresia in aging laying chickens through activation of PI3K/AKT and calcium signaling pathways. Oxid Med Cell Longev. 2020;2020:3648040.
Landry DA, Yakubovich E, Cook DP, Fasih S, Upham J, Vanderhyden BC. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci Adv. 2022;8:eabq1475.
Huang CC, Chou CH, Yang YS, Ho HN, Shun CT, Wen WF, Chen SU, Chen MJ. Metformin: a novel promising option for fertility preservation during cyclophosphamide-based chemotherapy. Mol Hum Reprod. 2021;27:gaaa084.
Guo Z, Yu Q. Role of mTOR signaling in female reproduction. Front Endocrinol (Lausanne). 2019;10:692.
Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–24.
Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410.
Sato Yorino, Kawamura K. Rapamycin treatment maintains developmental potential of oocytes in mice and follicle reserve in human cortical fragments grafted into immune-deficient mice. Mol Cell Endocrinol. 2020;504:110694.
Dou X, Sun Y, Li J, Zhang J, Hao D, Liu W, Wu R, Kong F, Peng X, Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16:825–36.
Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res. 2017;10:56.
Schafer MJ, Miller JD, LeBrasseur NK. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol. 2017;455:93–102.
Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–35.
Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93.
Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8:3–11.
Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–8.
Hense JD, Garcia DN, Isola JV, Alvarado-Rincón JA, Zanini BM, Prosczek JB, Stout MB, Mason JB, Walsh PT, Brieño-Enríquez MA, Schadock I, Barros CC, Masternak MM, Schneider A. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience. 2022;44:1747–59.
Gao Y, Wu T, Tang X, Wen J, Zhang Y, Zhang J, Wang S. Increased cellular senescence in doxorubicin-induced murine ovarian injury: effect of senolytics. Geroscience. 2023. https://doi.org/10.1007/s11357-023-00728-2
Du D, Tang X, Li Y, Gao Y, Chen R, Chen Q, Wen J, Wu T, Zhang Y, Lu H, Zhang J, Wang S. Senotherapy protects against cisplatin-induced ovarian injury by removing senescent cells and alleviating DNA damage. Oxid Med Cell Longev. 2022;2022:9144644.
Raghu G, Berk M, Campochiaro PA, Jaeschke H, Marenzi G, Richeldi L, Wen FQ, Nicoletti F, Calverley PMA. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr Neuropharmacol. 2021;19:1202–24.
Cao X, Guo L, Zhou C, Huang C, Li G, Zhuang Y, Yang F, Liu P, Hu G, Gao X, Guo X. Effects of N-acetyl-l-cysteine on chronic heat stress-induced oxidative stress and inflammation in the ovaries of growing pullets. Poult Sci. 2023;102:102274.
Fan L, Guan F, Ma Y, Zhang Y, Li L, Sun Y, Cao C, Du H, He M. N-Acetylcysteine improves oocyte quality through modulating the Nrf2 signaling pathway to ameliorate oxidative stress caused by repeated controlled ovarian hyperstimulation. Reprod Fertil Dev. 2022;34:736–50.
Fabbri R, Sapone A, Paolini M, Vivarelli F, Franchi P, Lucarini M, Pasquinelli G, Vicenti R, Macciocca M, Venturoli S, Canistro D. Effects of N-acetylcysteine on human ovarian tissue preservation undergoing cryopreservation procedure. Histol Histopathol. 2015;30:725–35.
Barrozo LG, Paulino LRFM, Silva BR, Barbalho EC, Nascimento DR, Neto MFL, Silva JRV. N-acetyl-cysteine and the control of oxidative stress during in vitro ovarian follicle growth, oocyte maturation, embryo development and cryopreservation. Anim Reprod Sci. 2021;231:106801.
Yosef B, Zhou Y, Mouschouris K, Poteracki J, Soker S, Criswell T. N-Acetyl-L-cysteine reduces fibrosis and improves muscle function after acute compartment syndrome injury. Mil Med. 2020;185(Suppl 1):25–34.
Zhu QY, Tai S, Tang L, Xiao YC, Tang JJ, Chen YQ, Shen L, He J, Ouyang MQ, Zhou SH. N-acetyl cysteine ameliorates aortic fibrosis by promoting M2 macrophage polarization in aging mice. Redox Rep. 2021;26:170–5.
Honma S, Tani I, Sakai M, Soma I, Toriyabe K, Yoshida M. Effect of N-acetyl cysteine on renal interstitial fibrosis in mice. Biol Pharm Bull. 2020;43:1940–4.
Ozakpinar OB, Maurer AM, Ozsavci D. Ovarian stem cells: from basic to clinical applications. World J Stem Cells. 2015;7:757–68.
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50.
Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21.
Green SH, Zuckerman S. Further observations on oocyte numbers in mature rhesus monkeys (Macaca mulatta). J Endocrinol. 1954;10:284–90.
Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34:756–60.
Ahmadian S, Mahdipour M, Pazhang M, Sheshpari S, Mobarak H, Bedate AM, Rahbarghazi R, Nouri M. Effectiveness of stem cell therapy in the treatment of ovarian disorders and female infertility: a systematic review. Curr Stem Cell Res Ther. 2020;15:173–86.
Sheikhansari G, Aghebati-Maleki L, Nouri M, Jadidi-Niaragh F, Yousefi M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed Pharmacother. 2018;102:254–62.
Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Tiras B, Seli E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020;12:10211–22.
Hosseinisadat R, Farsi Nejad A, Mohammadi F. Intra-ovarian infusion of autologous platelet-rich plasma in women with poor ovarian reserve: a before and after study. Eur J Obstet Gynecol Reprod Biol. 2023;280:60–3.
Funding
FUNCAP PS1-0186–00240.01.00/21; UNIFOR 60/2022; CAPES; CNPq; FAPERGS; NIH/NIA R56AG074499.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Cavalcante, M.B., Sampaio, O.G.M., Câmara, F.E.A. et al. Ovarian aging in humans: potential strategies for extending reproductive lifespan. GeroScience 45, 2121–2133 (2023). https://doi.org/10.1007/s11357-023-00768-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00768-8