Abstract
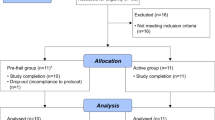
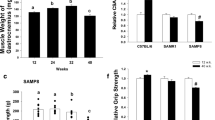
The interleukin-10 knockout mouse (IL10tm/tm) has been proposed as a model for human frailty, a geriatric syndrome characterized by skeletal muscle (SM) weakness, because it develops an age-related decline in SM strength compared to control (C57BL/6J) mice. Compromised energy metabolism and energy deprivation appear to play a central role in muscle weakness in metabolic myopathies and muscular dystrophies. Nonetheless, it is not known whether SM energy metabolism is altered in frailty. A combination of in vivo 31P nuclear magnetic resonance experiments and biochemical assays was used to measure high-energy phosphate concentrations, the rate of ATP synthesis via creatine kinase (CK), the primary energy reserve reaction in SM, as well as the unidirectional rates of ATP synthesis from inorganic phosphate (Pi) in hind limb SM of 92-week-old control (n = 7) and IL10tm/tm (n = 6) mice. SM Phosphocreatine (20.2 ± 2.3 vs. 16.8 ± 2.3 μmol/g, control vs. IL10tm/tm, p < 0.05), ATP flux via CK (5.0 ± 0.9 vs. 3.1 ± 1.1 μmol/g/s, p < 0.01), ATP synthesis from inorganic phosphate (Pi → ATP) (0.58 ± 0.3 vs. 0.26 ± 0.2 μmol/g/s, p < 0.05) and the free energy released from ATP hydrolysis (∆G ∼ATP) were significantly lower and [Pi] (2.8 ± 1.0 vs. 5.3 ± 2.0 μmol/g, control vs. IL10tm/tm, p < 0.05) markedly higher in IL10tm/tm than in control mice. These observations demonstrate that, despite normal in vitro metabolic enzyme activities, in vivo SM ATP kinetics, high-energy phosphate levels and energy release from ATP hydrolysis are reduced and inorganic phosphate is elevated in a murine model of frailty. These observations do not prove, but are consistent with the premise, that energetic abnormalities may contribute metabolically to SM weakness in this geriatric syndrome.





Similar content being viewed by others
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Balaban RS, Koretsky AP (2011) Interpretation of 31P NMR saturation transfer experiments: what you can’t see might confuse you. Focus on "Standard magnetic resonance-based measurements of the Pi–>ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles". Am J of Physiol Cell Physiol 301:C12–15
Barnes PR, Kemp GJ, Taylor DJ, Radda GK (1997) Skeletal muscle metabolism in myotonic dystrophy A 31P magnetic resonance spectroscopy study. Brain : A J of Neurol 120(Pt 10):1699–1711
Bittl JA, DeLayre J, Ingwall JS (1987) Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart, and skeletal muscle of the living rat. Biochemistry 26:6083–6090
Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI (2001) In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem 276:20240–20244
Conley KE, Amara CE, Bajpeyi S, Costford SR, Murray K, Jubrias SA, Arakaki L, Marcinek DJ, Smith SR (2013) Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary versus active subjects. J Clin Endocrinol Metab 98:129–136
Das AM, Steuerwald U, Illsinger S (2010) Inborn errors of energy metabolism associated with myopathies. J Biomed Biotechnol 2010:340849
Dzeja PP, Terzic A (2003) Phosphotransfer networks and cellular energetics. J Exp Biol 206:2039–2047
England K, Cotter TG (2005) Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Report : Comm in Free Radical Res 10:237–245
Feng J, Xie H, Meany DL, Thompson LV, Arriaga EA, Griffin TJ (2008) Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. The J of Gerontol Series A, Biol Sci and Med Sc 63:1137–1152
From AH, Ugurbil K (2011) Standard magnetic resonance-based measurements of the Pi → ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J of Physiol Cell Physiol 301:C1–11
Gibbs C (1985) The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol 17:727–731
Gupta A, Chacko VP, Schar M, Akki A, Weiss RG (2011) Impaired ATP kinetics in failing in vivo mouse heart. Circulation Cardiovascular Imaging 4:42–50
Gupta A, Akki A, Wang Y, Leppo M, Chacko VP, Foster D, Cicares V, Kirk J, Su J, Shi S, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG (2012) Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy-starved. J Clin Invest 122:291–302
Kanapuru B, Ershler WB (2009) Inflammation, coagulation, and the pathway to frailty. Am J Med 122:605–613
Kemp GJ (2008) The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 294:E640–642, author reply E643-644
Kemp GJ, Brindle KM (2012) What do magnetic resonance-based measurements of Pi → ATP flux tell us about skeletal muscle metabolism? Diabetes 61:1927–1934
Ko F, Yu Q, Xue QL, Yao W, Brayton C, Yang H, Fedarko N, Walston J (2012) Inflammation and mortality in a frail mouse model. AGE (Dordr) 34:705–715
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274
Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41:1234–1238
Nair KS (2005) Aging muscle. Am J Clin Nutr 81:953–963
Nuss JE, Amaning JK, Bailey CE, DeFord JH, Dimayuga VL, Rabek JP, Papaconstantinou J (2009) Oxidative modification and aggregation of creatine kinase from aged mouse skeletal muscle. Aging 1:557–572
Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 17:1688–1699
Posterino GS, Fryer MW (1998) Mechanisms underlying phosphate-induced failure of Ca2+ release in single skinned skeletal muscle fibres of the rat. J Physiol 512(Pt 1):97–108
Radda GK (1986) The use of NMR spectroscopy for the understanding of disease. Science 233:640–645
Ronner P, Friel E, Czerniawski K, Frankle S (1999) Luminometric assays of ATP, phosphocreatine, and creatine for estimation of free ADP and free AMP. Anal Biochem 275:208–216
Russ DW, Lanza IR (2011) The impact of old age on skeletal muscle energetics: supply and demand. Current Aging Sci 4:234–247
Saupe KW, Spindler M, Hopkins JC, Shen W, Ingwall JS (2000) Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. J of Biol Chem 275:19742–19746
Schrack JA, Simonsick EM, Ferrucci L (2010) The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc 58(Suppl 2):S329–336
Schuh RA, Jackson KC, Khairallah RJ, Ward CW, Spangenburg EE (2012) Measuring mitochondrial respiration in intact single muscle fibers. Am J Physiol Regul Integr Comp Physiol 302:R712–719
Staunton L, O’Connell K, Ohlendieck K (2011) Proteomic profiling of mitochondrial enzymes during skeletal muscle aging. J of Aging Res 2011:908035
Steenbergen C, Deleeuw G, Rich T, Williamson JR (1977) Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res 41:849–858
Ventura-Clapier R, Kaasik A, Veksler V (2004) Structural and functional adaptations of striated muscles to CK deficiency. Mol Cell Biochem 256–257:29–41
Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular HS (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162:2333–2341
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP (2006) Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54:991–1001
Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K (2008) The physical and biological characterization of a frail mouse model. J of Gerontol Series A, Biol Sci and Med Sci 63:391–398
Weiss RG, Gerstenblith G, Bottomley PA (2005) ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A 102:808–813
Wortmann RL (1991) Metabolic myopathies. Curr Opin Rheumatol 3:925–933
Younkin DP, Berman P, Sladky J, Chee C, Bank W, Chance B (1987) 31P NMR studies in Duchenne muscular dystrophy: age-related metabolic changes. Neurology 37:165–169
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Akki, A., Yang, H., Gupta, A. et al. Skeletal muscle ATP kinetics are impaired in frail mice. AGE 36, 21–30 (2014). https://doi.org/10.1007/s11357-013-9540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-013-9540-0