Abstract
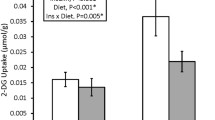
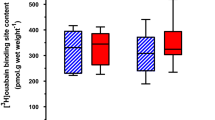
Exercise has been demonstrated to enhance subsequent insulin-stimulated glucose uptake (GU) by predominantly type II (fast-twitch) muscle of old rats, but previous research has not evaluated exercise effects on GU by type I (slow-twitch) muscle from old rats. Accordingly, we studied male Fischer 344/Brown Norway rats (24 months old) and determined GU (0, 100, 200, and 5,000 μU/ml insulin) of isolated soleus (predominantly type I) and epitrochlearis (predominantly type II) muscles after one exercise session. Epitrochlearis (100, 200, and 5,000 μU/ml insulin) and soleus (100 and 200 μU/ml insulin) GU were greater at 3-h postexercise vs. age-matched sedentary controls. Insulin receptor tyrosine phosphorylation (Tyr1162/1163) was unaltered by exercise in either muscle. Akt phosphorylation (pAkt) was greater for exercised vs. sedentary rats in the epitrochlearis (Ser473 and Thr308 with 100 and 200 μU/ml, respectively) and soleus (Ser473 with 200 μU/ml). AS160 phosphorylation (pAS160) was greater for exercised vs. sedentary rats in the epitrochlearis (Thr642 with 100 μU/ml), but not the soleus. Exercised vs. sedentary rats did not differ for total protein abundance of insulin receptor, Akt, AS160, or GLUT4 in either muscle. These results demonstrate that both predominantly type I and type II muscles from old rats are susceptible to exercise-induced improvement in insulin-mediated GU by mechanisms that are independent of enhanced insulin receptor tyrosine phosphorylation or altered abundance of important signaling proteins or GLUT4. Exercise-induced elevation in pAkt, and possibly pAS160, may contribute to this effect in the epitrochlearis of old rats, but other mechanisms are likely important for the soleus.





Similar content being viewed by others
References
Arias EB, Kim J, Funai K, Cartee GD (2007) Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292(4):E1191–E1200. doi:10.1152/ajpendo.00602.2006
Bonen A, Tan MH, Watson-Wright WM (1984) Effects of exercise on insulin binding and glucose metabolism in muscle. Can J Physiol Pharmacol 62(12):1500–1504
Bonen A, Tan MH, Clune P, Kirby RL (1985) Effects of exercise on insulin binding to human muscle. Am J Physiol 248(4 Pt 1):E403–E408
Bruss MD, Arias EB, Lienhard GE, Cartee GD (2005) Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54(1):41–50
Cartee GD, Bohn EE (1995) Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol 268(5 Pt 1):E902–E909
Cartee GD, Funai K (2009) Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37(4):188–195. doi:10.1097/JES.0b013e3181b7b7c500003677-200910000-00007
Cartee GD, Holloszy JO (1990) Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol 258(2 Pt 1):E390–E393
Cartee GD, Wojtaszewski JF (2007) Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32(3):557–566. doi:10.1139/H07-026
Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO (1989a) Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol 256(4 Pt 1):E494–E499
Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO (1989b) Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol 256(4):E494–E499
Cartee GD, Briggs-Tung C, Kietzke EW (1993) Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol 75(2):972–978
Catalano KJ, Bergman RN, Ader M (2005) Increased susceptibility to insulin resistance associated with abdominal obesity in aging rats. Obes Res 13(1):11–20. doi:10.1038/oby.2005.4
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30(12):1000–1007
Escriva F, Gavete ML, Fermin Y, Perez C, Gallardo N, Alvarez C, Andres A, Ros M, Carrascosa JM (2007) Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol 194(1):131–141. doi:10.1677/joe.1.07043
Facchini FS, Hua N, Abbasi F, Reaven GM (2001) Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86(8):3574–3578
Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M (2007) Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117(8):2289–2301. doi:10.1172/JCI31408
Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA (2002) Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282(1):E18–E23
Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD (2009) Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297(1):E242–E251. doi:10.1152/ajpendo.00194.2009
Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD (2010) In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab 298(5):E999–E1010. doi:10.1152/ajpendo.00758.2009
Gu Z, Du Y, Liu Y, Ma L, Li L, Gong Y, Tian H, Li C (2011) Effect of aging on islet beta-cell function and its mechanisms in Wistar rats. Age (Dordr). doi:10.1007/s11357-011-9312-7
Hamada T, Arias EB, Cartee GD (2006) Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol 101(5):1368–1376. doi:10.1152/japplphysiol.00416.2006
Hansen PA, Gulve EA, Holloszy JO (1994) Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76(2):979–985
Hansen PA, Nolte LA, Chen MM, Holloszy JO (1998) Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85(4):1218–1222
Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ (2002) Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes 51(2):479–483
Kahn CR (1978) Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metab Clin Exp 27(12 Suppl 2):1893–1902
Koshinaka K, Kawasaki E, Hokari F, Kawanaka K (2009) Effect of acute high-intensity intermittent swimming on postexercise insulin responsiveness in epitrochlearis muscle of fed rats. Metab Clin Exp 58(2):246–253. doi:10.1016/j.metabol.2008.09.021
Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ (2006) AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281(42):31478–31485. doi:10.1074/jbc.M605461200
Nishimura H, Kuzuya H, Okamoto M, Yoshimasa Y, Yamada K, Ida T, Kakehi T, Imura H (1988) Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol 254(1 Pt 1):E92–E98
Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI (1996) Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335(18):1357–1362. doi:10.1056/NEJM199610313351804
Richter EA, Garetto LP, Goodman MN, Ruderman NB (1982) Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69(4):785–793
Roach WG, Chavez JA, Miinea CP, Lienhard GE (2007) Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403(2):353–358. doi:10.1042/BJ20061798
Sakamoto K, Holman GD (2008) Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295(1):E29–E37. doi:10.1152/ajpendo.90331.2008
Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278(17):14599–14602. doi:10.1074/jbc.C300063200
Sharma N, Arias EB, Sajan MP, MacKrell JG, Bhat AD, Farese RV, Cartee GD (2010) Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J Appl Physiol 108(6):1631–1640. doi:10.1152/japplphysiol.01412.2009
Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K (2007) High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metab Clin Exp 56(12):1719–1728. doi:10.1016/j.metabol.2007.07.017
Thong FS, Derave W, Kiens B, Graham TE, Urso B, Wojtaszewski JF, Hansen BF, Richter EA (2002) Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 51(3):583–590
Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ (1999) Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol 277(4 Pt 1):E733–E741
Treadway JL, James DE, Burcel E, Ruderman NB (1989) Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol 256(1 Pt 1):E138–E144
Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ (1999) Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest 104(9):1257–1264. doi:10.1172/JCI7961
Wojtaszewski JF, Hansen BF, Gade KB, Markuns JF, Goodyear LJ, Richter EA (2000) Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49(3):325–331
Xie X, Gong Z, Mansuy-Aubert V, Zhou QL, Tatulian SA, Sehrt D, Gnad F, Brill LM, Motamedchaboki K, Chen Y, Czech MP, Mann M, Kruger M, Jiang ZY (2011) C2 Domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab 14(3):378–389. doi:10.1016/j.cmet.2011.06.015
Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM (2007) Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol 27(14):5172–5183. doi:10.1128/MCB.02298-06
Zorzano A, Balon TW, Garetto LP, Goodman MN, Ruderman NB (1985) Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol 248(5 Pt 1):E546–E552
Acknowledgments
This research was supported by the National Institutes of Health Grant AG-010026.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xiao, Y., Sharma, N., Arias, E.B. et al. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. AGE 35, 573–582 (2013). https://doi.org/10.1007/s11357-012-9383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-012-9383-0