Abstract
The aim of the study was to determine totoxicity of bisphenol A (BPA) and its derivatives (bisphenol S (BPS), bisphenol F (BPF), and tetrabromobisphenol A (TBBPA)) due to its high accumulation in environment. The performed analysis revealed the toxicity of the BPA, BPF, and BPS against Kurthia gibsoni, Microbacterium sp., and Brevundimonas diminuta as the most sensitive, reaching microbial toxic concentrations in the range of 0.018–0.031 mg ∙ L−1. Moreover, the genotoxicity assay shows the ability of all tested compounds to increase in the β-galactosidase level at the concentration range 7.81–500 µM (in Escherichia coli, PQ37). In turn, the matbolic activation of tested bishpenols has caused the enhacement of the genotoxicity and cytotoxicity effect. Interestingely, the highest phytotoxicity effect was pointed for BPA and TBBPA at the concentrations of 10 mg ∙ L−1 and 50 mg ∙ L−1, which cause the inhibition of root growth by 58% and 45%, respectively (especially for S. alba and S. saccharatum). Furthermore, the cytotoxicity analyses show the ability of BPA, BPS, and TBBPA to significantly decrease the metabolic activity of human keratynoctes in vitro after 24 h of treatment at the micromolar concentrations. Simialry, the impact of the certain bisphenols on proliferation-, apoptosis-, and inflammation-related mRNA expression was shown in tested cell line. Summarizing, the presented results have proved that BPA and its derrivatives are able to show high negative effect on certain living orgnisms such as bacteria, plants, and human cells, which is strict related to pro-apoptotic and genotoxic mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide production of BPA has gradually increased and reached 6.2 million tons (MT) in 2020, with predicted 7.1 MT by 2027 (Staples et al. 2010; Abraham and Chakraborty 2020). Humans are exposed to BPA via dietary and non-dietary sources, e.g., BPA is used in the production of polycarbonate plastics and epoxy resins, paper products (e.g., thermal receipts), toys, medical equipment, and electronics, as well as food plastic containers, whose damage causes BPA release into stored food (Filardi et al. 2020). BPA is characterized by lipophilic properties, which facilitates penetration of biological membranes and cell interiors, and accumulation in adipose tissue (Fernandez et al. 2007). This specific property also allows to overcome both the placental barrier and blood–brain barrier; therefore, BPA and its derivatives accumulate in the fetal compartment after repeated maternal exposure (Doerge et al. 2011; Grandin et al. 2018; Filardi et al. 2020). Different concentrations of bisphenols, mainly BPA, have been detected in urine (0.45–0.73 ng mL−1) and serum (0.5–16 ng mL−1) in individuals of different ages, occupations, and gender around the world, with higher level of BPA in serum was observed in exposed workers (0.95–3.81 ng mL−1 and 0.48–3.19 ng mL−1, respectively) (Ribeiro-Varandas et al. 2013; Bousoumah et al. 2021; Xing et al. 2022).
BPA is released into the environment with effluents from wastewater treatment plants associated with the use of BPA in polycarbonate/epoxy resins (Melcer and Klecka 2011) and in untreated landfill leachates (Masoner et al. 2014). The concentration of bisphenols detected in the aquatic environment often exceeds 1500 ng L−1 recommended by the European Union as the predicted no-effect concentration (PNEC) (EU 2008). Bisphenol concentrations in industrial areas in Korea, China, Japan, and India can reach several times higher values (up to 7200 ng L−1) than in other regions (Morales et al. 2020; Cosentino et al. 2022). Bisphenols are mostly detected in the aquatic environment, where, depending on analytical detection methods, their concentrations are in the range of 1260–106,900 ng L−1 (lake water), 1800–107,700 ng L−1 (river water), and 5.3–128 ng L−1 (drinking water) (Gallart-Ayala et al. 2010; Yin et al. 2011; Yang et al. 2014; Shan et al. 2014; Caballero-Casero et al. 2016; Abraham and Chakraborty 2020).
Due to increased concerns regarding BPA toxicity to the environment and living organisms, its several analogs are currently being introduced as substitutes, e.g., bisphenol F (BPF), bisphenol S (BPS), and tetrabromobisphenol-A, a brominated derivate of BPA (TBBPA) (Szychowski and Wójtowicz 2013; Chen et al. 2016; Qiu et al. 2019). BPS and BPF replace BPA in many products, but TBBPA, due to its flame-retardant properties, is used as an additive to textiles, furniture, appliances, and electronic devices, especially those used in the aviation industry. Moreover, TBBPA, BPA, and its derivates are released from plastics during their use (Kousaiti et al. 2020; Holmes et al. 2021). However, these BPA derivatives also raise concerns regarding their toxicity to human health and widespread occurrence in the environment via bioaccumulation and biomagnification (Ruan et al. 2015; Chen et al. 2016; Wang et al. 2017; Zhang et al. 2018b). Recent studies have indicated that similarly to BPA, its derivatives also exert adverse effects on human health as EDs and can be accumulated in adult and fetal human tissues (Chen et al. 2016). To date, a number of health safety studies involving bisphenol analogs have shown their various toxic effects, including cytotoxicity, neurotoxicity, reproductive toxicity, genotoxicity, and carcinogenicity (Mustieles and Fernández 2020; Xing et al. 2022). Moreover, TBBPA can be microbiologically transformed into BPA in the natural environment; thus, its biologically active intermediate metabolites are still present in the environment (Szychowski and Wójtowicz 2013).
In addition to the effect on human health, BPA and its derivatives (BPS, BPF, and TBBPA) also impair the microbiological and biochemical soil balance, as well as the growth and development of plants (Xie et al. 2018; Zaborowska et al. 2019, 2020a, b, 2021; Xiao et al. 2020). Bisphenols enter agricultural soil mainly from sewage sludge and effluents from wastewater treatment plants used for agriculture irrigation, which are characterized by a significant content of these xenoestrogens (2.16–1.740 pg g−1 dw and 0.24–696 ng L−1, respectively) (Lee et al. 2015; Caballero-Casero et al. 2016; Pérez et al. 2017; Liu et al. 2021). The biotic stress triggered by the presence of bisphenols in soil causes varied responses of soil microorganisms, and thus their soil enzyme activities (Zaborowska et al. 2021). Zaborowska et al. (2019; 2020a, 2020b, 2021) indicated that BPA, BPF, and BPS interfered with the growth of soil microorganisms, especially bacteria, and disturbed their homeostasis by inhibiting enzymatic activity (Zaborowska et al. 2019, 2020a, 2021).
The aim of our study was to determine the bio-, phyto-, and genotoxicity of BPA and its derivatives (BPS, BPF, and TBBPA). Among human tissues, skin is most likely to be exposed to the external environment. Therefore, the cytotoxicity and molecular mechanism of action of these xenoestrogens on human keratinocyte HaCaT cells was also determined by evaluating the expression of genes responsible for cell growth and proliferation (Ki67, ATM, p53) and oxidative stress (SOD2, CAT, SHH, PPARγ, NF-κB1, NRL2L2).
Materials and methods
Chemicals
Bisphenol A (cat. 239658), bisphenol F (cat. 51453), bisphenol S (cat. 43034), tetrabromobisphenol A (cat. 330396), trypsin, penicillin/streptomycin, and resazurin sodium salt were purchased from Sigma-Aldrich (Germany). Phosphate-buffer saline (PBS) and Dulbecco’s modified Eagle medium (DMEM) without phenol red were obtained from Corning (USA). Charcoal–dextran treated fetal bovine serum (FBS), Gene MATRIX Universal RNA Purification Kit, and Fast Probe qPCR Master Mix (2x) plus ROX Solution were purchased from EURx (Poland). The High-Capacity cDNA Reverse Transcription Kit, TaqMan probes and primers complementary to genes encoding ACTB (Hs01060665_g1), KI67 (Hs04260396_g1), TP53 (Hs01034249_m1), ATM (Hs00175892_m1), SHH (Hs00179843_m1), SOD2 (Hs00167309_m1), CAT (Hs00156308_m1), PPARG (Hs00234592_m1), NFL2E2 (Hs00975961_g1), and NFKB1 (Hs00765730_m1) were purchased from Thermo Fisher Scientific (USA). The MARA assay, Phytotoxkit, and SOS Chromotest were purchased from Tigret (Tigret, Poland distribution).
The tested compounds were purchased at the highest possible purity (certificates of analysis available at the producer’s website — Sigma-Aldrich) as a powder, which subsequently were weight and dissolved in the DMSO to obtain 1000-time concentrated stocks. These stocks were subsequently used in the analysis with the following bisphenol dilutions ranges: 0.0156–1 mg L−1 (MARA toxicity assay); 10 and 50 mg L−1 (Phytotoxkit assay), 7.81–500 µM (genotoxicity SOS Chromotest), and 1 nM–100 µM (human cell culture studies: resazurin reduction assay and qPCR). Dilutions of bisphenols tested in the present study were selected on the basis of literature data and optimization experiments.
Assessment of toxicity using multi-species microbial assay (MARA)
The multi-species microbial assay (MARA) was used to toxicity of bisphenol derivatives, respectively. In the MARA assay, lyophilized microorganisms placed in row H of the microplates were rehydrated and pre-incubated for 4 h at 30 °C. Series of six dilutions of the initial solution of each bisphenol derivatives (1, 0.5, 0.125, 0.0625, 0.0312, and 0.0156 mg L−1) were placed in rows G-B of the microplates. Pure medium was introduced into row A as a strain control. Subsequently, microorganisms from row H were added to each sample dilution. Microplates were incubated at 30 °C (18 h) and subsequently scanned in a flatbed scanner (Epson Perfection V550 Photo). The results were processed using an image analysis program that facilitates calculation of the average MTC and minimum MTC (microbial toxic concentration) values (mg L−1). Moreover, based on the growth inhibition results for each strain, the EC50 value (half-maximal effective concentration) was calculated for each bisphenol and classified as: EC50 < 1 mg L−1 — very toxic to aquatic organisms, 1–10 mg L−1 — toxic to aquatic organisms, 10–100 mg L−1 harmful to aquatic organisms, and > 100 mg L−1 — not classified as harmful to aquatic organisms (European Commission (EC) 1993).
Assessment of genotoxicity
For the SOS ChromoTest assessing genotoxicity, overnight bacterial cultures were grown in fresh LB medium to an optical density (OD600nm) of 0.5–0.6, diluted tenfold in double strength LB medium (20 g tryptone L−1, 10 g yeast extract L−1, 20 g sodium chloride L−1, pH 7.4), and mixed (v/v) with the tested compounds, e.g., potential mutagens (or promutagens when lyophilized rat liver S9 fraction with cytochrome P450 activity was added) and solvents. As positive controls, 4-nitroquinoline 1-oxide (4NQO), in the range from 0.078 to 10 µg mL−1 (without metabolic activation by S9 fraction), and 2-aminoanthracene (2AA) (with metabolic activation by S9 fraction), in the range from 0.78 to 100 µg mL−1, were used. A negative control (distilled water) was included in each assay. Bacteria were exposed to different concentrations of bisphenol derivatives (7.81, 15.62, 31.25, 62.50, 125, 250, and 500 µM) for 1.5 h at 37 °C. The colorimetric reactions for β-galactosidase (β-gal) and alkaline phosphatase (AP) were estimated spectrophotometrically in 96-well plates at A600nm and A420nm. Significant genotoxic activity was defined as an adjusted induction factor (CIF) equal to or greater than 1.2.
Phytotoxicity assessment
Phytotoxkit (Tigret, Poland) was used to determine the direct effects of bisphenol derivatives (at concentrations of 10 and 50 mg L−1) on the germination and growth of young roots of Lepidium sativum, Sinapis alba, and Sorgoum saccharatum compared to controls (distilled water) in reference soil. The germination index (GI) and root growth inhibition (RGI) of seeds exposed to bisphenols were assessed and compared with germination and growth of control, according to the manufacturer’s protocol.
In vitro toxicity to human cells
Cell culture and treatment
The human keratinocyte cell line (HaCaT; ATCC CRL-2404) was obtained from the American Type Culture Collection (ATCC, distributor: LGS Standard, Łomianki, Poland). The cells were cultured in DMEM without phenol red, supplemented with 10% FBS and penicillin and streptomycin, in a humidified atmosphere with 5% CO2 at 37 °C until confluence. Subsequently, the cells were trypsynized and seeded in 96-well plates at a density of 4.5 × 103 cells/well or in 12-well plates at a density of 9 × 104 cells/well for the resazurin reduction assay and real-time PCR, respectively. After 24 h, the medium was removed and replaced with fresh one containing charcoal–dextran-treated FBS (known to be free of steroid hormones and various other substances) and increasing concentrations (in the range from 1 nM to 100 µM) of BPA, BPF, BPS, and TBBPA.
Resazurin reduction assay
The resazurin reduction assay was performed according to the protocol of Ivanov et al. (Ivanov et al. 2014). Briefly, the cells were seeded as described above and initially cultured for 24 h. Next, the medium was replaced with fresh one containing increasing concentrations (1 nM–100 µM) of BPA, BPF, BPF, and TBBPA for 24 h. Subsequently, the medium was removed and new one with 1% resazurin was added to each well for 1 h. After this time, the measurements were performed at λex. = 530 nm and λem. = 590 nm using a microplate reader (FuilterMax F5). The results were normalized to the vehicle-treated cells and expressed as percentage (%) of control.
Real-time polymerase chain reaction (RT-PCR) in HaCaT cells
Real-time PCR was performed as described by Skóra et al. (2022). Briefly, the cells were seeded in 12-well plates 24 h before the experiment. After this time, the medium was replaced with fresh one containing 50 µM BPA, BPF, BPF, and TBBPA for 24 h. Subsequently, isolation of total RNA was performed, using the Gene MATRIX Universal RNA Purification Kit according to the producer’s manual (EURx, Poland). The quantity and quality of the RNA were assessed spectrophotometrically at 260/280 nm (ThermoFisher NanoDrop, USA). A reverse transcription reaction was then performed using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher, USA). Subsequently, the obtained cDNA template (1 µL) was used in real-time PCR together with the Fast Probe qPCR Master Mix (2x), plus ROX Solution (EURx), primers and TaqMan probes complementary to the sequences coding for the ACTB, KI67, TP53, ATM, SHH, SOD2, CAT, PPARG, NFL2E2, and NFKB1 genes; total reaction volume was always 20 µL. The qPCR program used in this study was as follows: 2 min at 50 °C and 10 min at 95 °C, followed by 45 cycles of 15 s at 95 °C and 1 min at 60 °C. The threshold value (Ct) for each sample was calculated during the exponential phase, and ΔΔCt was used to determine the average fold expression changes of the genes tested. ACTB was used as the reference gene.
Statistical analysis
The data are presented as means ± SD of three independent experiments (n = 3). The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test ***P < 0.001, **P < 0.01, and *P < 0.05 compared to control.
Results
Biotoxicity
The wide sensitivity range of the MARA species was observed in the presence of BPA, BPF, and BPS, with the most sensitive species in the order of: Kurthia gibsoni, Microbacterium sp., and Brevundimonas diminuta (MTC min. = 0.018–0.031 mg L−1) (Fig. 1). The BPA, BPF, and BPS samples were characterized by very low average MTC values ranging from 0.018 to 0.12 mg L−1, classified as toxic compounds. The TBBPA sample was characterized by low selective toxicity, with the most sensitive strain being Kurthia gibsoni (MTC min. = 0.13 mg L−1). Pseudomonas aurantiaca was characterized by the highest resistance to BPA, TBBPA, and BPF of the strains included in the MARA assay (MTC max. = 0.95–0.98 mg L−1). The highest resistence in the presence of BPS was recorded for Enterococcus casseliflavus (MTC max. = 0.97 mg L−1) (Fig. 1). Moreover, the obtained EC50 values classified BPA, BPS, BPF, and TBBPA as very toxic compounds (Table 1).
Microbial toxic concentration (MTX in mg/L) of BPA and its derivatives for each strain (1) Microbacterium sp., (2) Brevundimonas diminuta, (3) Citrobacter freudii, (4) Comamonas testosteroni, (5) Entrococcus casseliflavus, (6) Delftia acidovorans, (7) Kurthia gibsoni, (8) Staphylococcus warneri, (9) Pseudomonas aurantiaca, (10) Serriatia rudidaea, and (11) Pichia anomala
Genotoxicity
The results showed high genotoxicity of the initial BPA, TBBPA, BPF, and BPS solutions and their twofold dilution (7.81–500 µM), with the CIF factor ranging from 2.63 to 4.03. The genotoxicity of bisphenols in the presence of S9 fraction was indicated by CIF = 0.89–1.75, with a higher CIF for 7.81–250 µM concentrations (Table 2).
Phytotoxicity
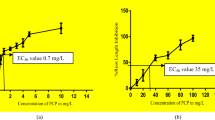
The addition of bisphenols (BPS and BPF) did not inhibit seed germination of the test plants, and GI of S. alba was slightly inhibited only by 50 mg L−1 BPA and TBBPA (70 and 90%, respectively; Figure S1). The phytoxicity test showed the effect of bisphenols mainly on the root growth of L. sativum, S. alba, and S. saccharatum plants. For all test plants, a significant root growth inhibition was recorded in the presence of BPA, TBBPA, BPF, and BPS at concentrations of 10 and 50 mg−1, with the highest RGI = 58% and 44.62% in the presence of BPA and TBBPA at 50 mg L−1, determined for S. alba and S. saccharatum, respectively (Fig. 2).
Phytotoxicity of BPA and its derivatives to Lepidium sativum L (A), Sinapis alba (B) and Sorgoum saccharatum (C); RGI, root growth inhibition; *, *** and #, ### indicate statistically significant differences between bisphenols at concentrations of 10 mg L−1 and 50 mg L.−1 at p < 0.05 and p < 0.001, respectively (one-way ANOVA, Tukey’s test)
Cytotoxicity and metabolism of HaCaT cells
Metabolic activity assay
After 24 h of treatment, only 100 µM BPA caused a decrease in metabolic activity (by 16.39%) compared to control (Fig. 3A). No changes were observed for the other tested BPA concentrations in HaCaT cells (Fig. 3A). HaCaT cells treated with BPS for 24 h showed a decrease in metabolic activity compared to control: 20.35, 24.20, 16.04, and 31.30% for 1, 10, 50, and 100 µM BPS, respectively (Fig. 3C). In turn, only the highest TBBPA concentration caused a decrease in metabolic activity (by 19.32%) after 24-h treatment compared to control (Fig. 3D).
Metabolic activity of HaCaT cells treated with BPA (A), BPF (B), BPS (C), and TBBPA (D) for 24 h. Mean values (n = 3) with standard deviation (error bars) marked with *, **, and *** are statistically different from the respective (vehicle-treated) control at p < 0.05, p < 0.01, and p < 0.001, respectively (one-way ANOVA, Tukey’s test)
Expression of genes responsible for growth and proliferation (Ki67, ATM, p53) and oxidative stress (SOD2, CAT, SHH, PPARg, NFKβ1, NRL2L2) in HaCaT cells
After 24-h treatment of HaCaT cells, mRNA expression of KI67 decreased by 27.45, 22.71, 42.76, and 35.04% compared to control in cells treated with BPA, BPS, BPF, and TBBPA, respectively (Fig. 4A). On the other hand, an increase in TP53 gene expression was observed in HaCaT cells treated with BPA and BPF (by 20.90 and 37.14%, respectively) (Fig. 4B). All tested compounds caused higher ATM mRNA expression by 118.61, 138.99, 217.19, and 89.29% for BPA, BPS, BPF, and TBBPA, respectively, compared to control (Fig. 4C). Moreover, HaCaT cells treated with BPA and BPF showed a 21.22 and 11.53% decrease in SOD2 expression, while TBBPA caused elevated mRNA expression by 44.91% compared to control (Fig. 4E). BPA-treated cells were characterized by a decrease in CAT mRNA expression by 56.01% compared to control, unlike BPS, which was able to increase this gene expression by 33.47% compared to control (Fig. 4F). The expression of PPARγ mRNA increased only in BPF-treated cells by 37.80%, while TBBPA caused a decrease in this gene expression by 29.83% compared to control (Fig. 4G).
Expression of the KI67 (A), TP53 (B), ATM (C), SHH (D), SOD2 (E), CAT (F), PPARγ (G), NFE2L2 (H), and NF-κB (I) genes in HaCaT cells treated with BPA, BPS, BPF, and TBBPA for 24 h. Mean values (n = 3) with standard deviation (error bars) marked with *, **, and *** are statistically different from the respective (vehicle-treated) control at p < 0.05, p < 0.01, and p < 0.001, respectively (one-way ANOVA, Tukey’s test). ACTB was used as a reference gene; nd, gene expression not detected
Discussion
The presence of bisphenol A and its derivatives in water and soil contributes to the disturbance of the biological balance of microorganisms and plants. The microbial strains used in the MARA assay belong to typical environmental strains naturally occurring in the soil and aquatic environment (Gabrielson et al. 2003; Bisht et al. 2010). Individual strains show different sensitivity to different chemicals and the obtained inhibition values provide a toxic fingerprint of the chemicals tested based on a panel of assays within a single test. Some of these strains, such as Pseudomonas sp., Microbacterium sp., Comamonas sp., or Delftia sp. are involved in the biodegradation of xenobiotics, e.g., phenols, polychlorinated bis-phenyls, and per- and polyfluorinated alkyl substances and pesticides (Singh et al. 2003; Abdel-Razek et al. 2015; Braña et al. 2016; Mishra et al. 2021; Harris et al. 2022). Moreover, previous data have indicated that rhizosphere bacteria, e.g., Kurthia sp., participate in naphthalene and anthracene decomposition (Bisht et al. 2010), and Kurthia sp. and Delphia sp. have the ability to promote plant growth (Bisht et al. 2010; Braña et al. 2016). Associative interactions of plants and microorganisms in soil bioremediation offer great opportunities because a suitable rhizosphere strain can be introduced together with an appropriate plant, thereby enhancing the bioremediation process. Moreover, bacteria that effectively colonize roots and decompose contaminants exploit growing roots that act as an injection system to spread the bacteria in soil (Bisht et al. 2010). Therefore, the toxic effect of xenobiotics against bacterial strains involved in the biodegradation of xenobiotics significantly reduces bioremediation efficiency of the microflora of the soil and water environment (Mishra et al. 2021). In accordance with the European Directive EC 93/67/EEC (European Commission (EC) 1993), chemicals are classified on the basis of their EC50 values — those with EC50 < mg L−1 are considered very toxic to aquatic organisms, 1–10 mg L−1 are toxic to aquatic organisms, 10–100 mg L−1 are harmful to aquatic organisms, and > 100 mg L−1 are not classified as harmful to aquatic organisms. This study showed that BPA, BPF, BPS, and TBBPA were characterized by acute toxicity for the tested microorganism, especially for Kurthia gibsoni, Microbacterium sp., and Brevundimonas diminuta, and based on European Directive EC 93/67/EEC, they should be classified as very toxic to aquatic organisms. On the other hand, previous studies have indicated that soil contamination with BPA, BPF, BPS, and TBBPA also poses a potential ecological risk to soil microbial community (Xie et al. 2018; Zaborowska et al. 2019, 2020b, 2021). Sources of BPA to soils include the application of sewage sludge (Langdon et al. 2012), irrigation with wastewater effluent (Chen et al. 2011), and amended with biosolids (Staples et al. 2010). The half-life of BPA in soil ranging from 3 to 37.5 days (Careghini et al. 2015) or even 75 days (Corrales et al. 2015). BPA concentrations in soil depending on the amount and type of effluent or waste received (Li et al. 2021; Dueñas-Moreno et al. 2022). Soils specifically treated with sewage sludge, irrigated with wastewater, and amended with biosolids contained BPA concentrations: 1–150 μg kg−1 (Langdon et al. 2012), 1.60–30.20 μg kg−1 (Gibson et al. 2010), and 0.25–1.15 μg kg−1 (Staples et al. 2010). Although the presence of BPA in agricultural fields irrigated with wastewater effluent is less than 10 μg kg−1 (Chen et al. 2011), Xie et al. (2018) indicated that growth inhibition of Chloroflexi (3.5–8.1%) and Actinomycetes (6.7–8.0%) was previously observed under aerobic and anaerobic conditions in the soil exposed to TBBPA (40 mg kg−1), but interestingly, TBBPA at this dose promoted the growth of Proteobacteria (35.7–41%), Bacteroidetes (only aerobic condition, 6.4%) and Acidobacteria (only under anaerobic conditions, 15.6%). This was consistent with our results, which indicated that Pseudomonas auriantiaca (ɤ-proteobacteria) was the most resistant strain to TBBPA contamination. Zaborowska et al. (2019, 2020a, 2020b, 2021) reported that BPA, BPF, and BPS inhibited the growth of soil microorganisms and disturbed their biochemical properties. Moreover, BPA, BPF, and BPS impaired the bioremediation potential of soil microorganisms (Zaborowska et al. 2019, 2020b, a).
In addition to the ecological balance of soil microorganisms, proper plant growth is also very important. Bisphenol A and its derivatives, in addition to disrupting the biodiversity of microorganisms in the water and soil environment, can also adversely affect plant germination and root growth (Dogan et al. 2010; Tian et al. 2014; Zhang et al. 2018a). The presence of xenoestrogens such as BPA and its derivatives in soil can significantly affect the germination and growth of crops and industrial plants, and disturb their phytoestrogen balance (Wang et al. 2015; Kim et al. 2018; Li et al. 2018a, b; Xiao et al. 2020). As described previously, BPS in soil are unstable; thus, changes in soil environmental conditions can cause the release of BPS and its metabolites from physicochemical entrapment and their subsequent accumulation in plants (Cao et al. 2020).
Seed germination is the starting point of plant life when the radicle breaks through the seed coat and develops into a new plant (Xiao et al. 2020). Previous studies indicated that different BPA concentrations can exert various effects on seed germination, e.g., BPA concentration ˃ 50 mg L−1 inhibited seed germination of Cicer arietinum (chickpea) and Arabidopsis thaliana (Dogan et al. 2010; Tian et al. 2014). This could be related to the inhibition of energy metabolism by higher BPA doses during seed germination (Xiao et al. 2020). In the case of chickpea, TBBPA also inhibited seed germination, but only at a dose of 100 mg L−1 (Dogan et al. 2010), while lower BPA concentrations (10 and 50 mg L−1) did not affect seed germination of these plants (Dogan et al. 2010; Tian et al. 2014). Moreover, the germination rates of various species treated with BPA differed, indicating that BPA tolerance among seeds of various plant species varied (Xiao et al. 2020).
Root growth is another parameter estimating the direct effects in plants exposed to xenobiotics. Previous studies have shown that BPA and its derivatives, depending on the exposure dose, can have different effects on plant root growth, usually induced oxidative stress (Dogan et al. 2010; Tian et al. 2014; Zhang et al. 2018a; Xiao et al. 2020; Ali et al. 2022). The production of reactive oxygen species (ROS) is a general primary response of plants to stress (Zhang et al. 2018a, b) and may involve self-protection responses of plants to stress induced by BPA exposure (Dogan et al. 2010; Zhang et al. 2018a, b). Previous studies showed that low BPA levels (1.5–50 mg L−1) induced ROS production in Glycine max (soybean) and Cicer arietinum (chickpea) roots. These results indicate that ROS in roots may be involved in the oxidative metabolism of BPA, which may prevent BPA damage to exposed plants. Exposure to low doses of BPA was also shown to enhance mitochondrial energy production in root cells (through the Krebs Cycle) and key enzyme activities in root respiration (Zhang et al. 2018a, b; Xiao et al. 2020). Zhang et al. (2018a, b) and Dogan et al. (2010) argued that longer BPA exposure led to higher ROS production, which in turn probably induced the expression of antioxidant enzymes degrading BPA (Dogan et al. 2010; Zhang et al. 2018a). On the other hand, Li et al. (2018a) indicated that BPA exposure at doses 6 and 17.2 mg L−1 could inhibit roots growth of Glycine max L. at 14.4 and 34.7%, respectively (Li et al. 2018a).
In addition to acute toxicity, it is also important to determine the long-term effects of xenobiotics, e.g., genotoxicity. While food safety and health organizations consider BPA a class 2 B reproductive toxin, the available data do not show a clear link between BPA and genotoxicity and carcinogenicity (Jalal et al. 2018; Ramos et al. 2019). Previous studies have not clearly confirmed BPA’s genotoxicity, but some of them indicated that BPA directly interfered with the mechanisms of cell division (George et al. 2008) and expression of genes involved in mitotic processes (Ribeiro-Varandas et al. 2013). Some data indicated genotoxic effects of BPA associated with decreased cellular proliferation at concentrations ≥ 100 μM (Bolli et al. 2008). However, our research showed that although the CIF factor was lower after metabolic activation using rat liver S9 fraction, bisphenols were still genotoxic. As previously described, microsomal cytochrome P450 enzymes in the liver can efficiently metabolize BPA via the glucuronidation and sulfation pathways; however, the bisphenol-o-quinone intermediate has been shown to be toxic and act as a DNA adduct (Kang et al. 2006; Jalal et al. 2018). Food and beverages are the main source of exposure to BPA and its derivatives into the human body (Genuis et al. 2012). However, the second largest source of human BPA and its derivatives exposure after food and beverage packaging is thermal paper (cash receipts, parking, airline and cinema tickets, luggage tags, bus and train tickets, and grocery weight tickets) (Reale et al. 2021). Therefore, we have examined the effect of BPA, BPS, BPF, and TBBPA cytotoxicity on HaCaT cells, as well as their impact on the expression of proliferation- and oxidative stress-related genes. Our results showed that after 24-h exposure of HaCaT cells to the test compounds, BPF did not significantly affect cell metabolism, while BPA and TBBPA inhibited resazurin reduction only at the highest, micromolar concentrations. Interestingly, BPS decreases the values of the resazurin reduction assay over a wide range of micromolar concentrations (1–100 µM). Previous data indicated that BPA could significantly affect the activity of HaCaT cells by activating DNA damage marker protein and inducing cell apoptosis. To date, the toxic effects of BPA have been well-described in various types of cell cultures and aquatic organisms, as well as mammals such as mice and rats (Jeong et al. 2013; Hwang et al. 2013; Abdel-Tawwab and Hamed 2018; Apaydin et al. 2019). Toxicity have been also reported for BPA derivatives such as BPF and BPS. Hercog et al. (2019) found that after 72 h of exposure of human hepatocellular carcinoma (HepG2) cells to 20 µg/mL BPA, BPS, and BPF, only BPS and BPF significantly reduced cell viability (Hercog et al. 2019). The latter authors reported that BPA also slightly decreased cell viability, but this effect was not statistically significantly. Huang et al. (2020) showed that 100 µM BPA, BPS, and BPF decreased the viability of the human granulosa (KGN) cell line (Huang et al. 2020). Interestingly, 100 nM BPS and BPF was shown to increase cell viability, which could also suggest their higher proliferation. In addition, TBBPA was also described to reduce cell viability in the broad range of concentrations, starting from 1 to 10 µM in mouse cortical and hippocampal neurons, 5 µM in human hepatocytes (L02), and 40 µM in the RAW264.7 cell line to 100 µM in the human choriocarcinoma-derived placental JEG-3 cell line (Wojtowicz et al. 2014; Honkisz and Wójtowicz 2015; Szychowski and Wójtowicz 2016; Park et al. 2019; Zhang et al. 2019). Therefore, our data are consistent with those described by other groups and indicate that HaCaT cells are relatively resistant to TBBPA-induced toxicity.
Based on the data from our research and literature reports, we have selected BPA, BPS, BPF, and TBBPA at 50 µM concentrations for further study. Our data showed that all compounds decreased the expression of the KI67 gene, which is a well-established indicator of cell proliferation (Miller et al. 2018). Similarly, all studied compounds increased ATM mRNA expression, i.e., a recognized marker of cell stress and DNA damage (Mazan-Mamczarz et al. 2011). However, only BPA and BPF increased the expression of TP53 mRNA, suggesting an initiated process of apoptosis. Our data showed that BPA and BPF reduced SOD2 mRNA expression, while TBBPA increased it in the HaCaT cell line. Moreover, our experiments showed that BPA reduced the levels of CAT mRNA transcript, while increased CAT mRNA expression was observed after BFS treatment. In our study, we did not detect SHH transcripts in HaCaT cells.
To date, only one article has described the role of ATM in the mechanism of bisphenol action (Mahemuti et al. 2018). In normal human fetal lung fibroblasts (HFLF), 100 µM BPA has been reported to strongly increase the expression of ATM-positive cells (Mahemuti et al. 2018). Higher expression of TP53 mRNA and/or protein levels by BPA is well documented in a number of cell types. Loyd et al. (2019) found that BPA increased TP53 protein expression in the breast carcinoma T-47D cell line at a concentration range of 200 nM to 2 µM, in and in the breast adenocarcinoma MCF-7 cell line at a concentration range of 1–2 µM (Lloyd et al. 2019). Similar results were obtained by Lucendo-Villarin et al. (2020), who showed that BPA, BPS, and BPF increased TP53 mRNA expression in the human pluripotent stem cell lines (Man12 (male)) (Lucendo-Villarin et al. 2020). Consistent results regarding elevated TP53 protein expression were also acquired in the mouse-derived C17.2 multipotent neural stem cell line treated with TBBPA at concentrations ranging from 12.5 to 50 µM (Cho et al. 2020). Moreover, contrary to our results, Hercog et al. (2019) showed that BPA, BPS, and BPF, at concentrations of 10 ng mL−1 and 10 µg mL−1, did not alter mRNA expression of GPX1, SOD1A, CAT, and TP53 antioxidant enzymes in HepG2 cells; however, the latter authors tested lower concentrations of bisphenols; therefore, the effect could be dose-dependent (Hercog et al. 2019). Huang et al. (2020) reported that BPA, BPS, and BPF reduced CAT, SOD, and GSH activities at a wide range of concentrations (between 1 and 100 µM) in human granulosa KGN cells (Huang et al. 2020). Similar to bisphenols, 1.00 mg L−1 TBBPA decreased the expression of SOD1, CAT, and GPX1A mRNAs expression in embryos and larvae of zebrafish (Danio rerio) (Wu et al. 2016). On the other hand, TBBPA concentrations from 5 to 40 µM increased CAT mRNA expression in L02 cells (Zhang et al. 2019). Therefore, we could conclude that our data were largely consistent with previous reports and showed that the selected bisphenol analogs exerted genotoxic (as demonstrated by increased ATM gene expression) and antiproliferative activity (proved by KI67 mRNA expression).
Nuclear factor erythroid 2-like 2 (NFE2L2) is a transcription factor that controls the cellular defense system against toxic and oxidative damage through the expression of genes involved in the oxidative stress response and drug detoxification (He et al. 2020). Similarly, peroxisome proliferator-activated receptor gamma (PPARγ) and sonic hedgehog (SHH) are involved in responses to xenobiotics, oxidative stress, cell metabolism, apoptosis, and cell differentiation (Janani and Ranjitha Kumari 2015; Lee et al. 2018; Lv et al. 2018). Therefore, to elucidate the mechanism of action of BPA, BPS, BPF, and TBBPA, we decided to analyze the levels of PPARγ, NFE2L2, NF-κB, and SHH mRNA transcripts. Our study demonstrated that BPF increased PPARγ mRNA level, while TBBPA reduced it. However, only BPF increased the expression of NFE2L2 mRNA. The current study showed that all test compounds were able to modify the level of NF-κB transcripts, while no SHH mRNA expression was detected in HaCaT cells. Interestingly, Gao et al. (2020) reported that 100 µM BPA and BPS increased PPARγ mRNA levels in the human macrophage THP-1 cell line (Gao et al. 2020), while 25 µM BPA and BPS was sufficient to increase PPARγ mRNA expression in murine 3T3-L1 preadipocytes (Ahmed and Atlas 2016); however, the latter authors did not observe changes in PPARγ mRNA at lower concentrations (0.01 to 10 µM) of the studied compounds (Ahmed and Atlas 2016). In human adipose-derived stem cells, BPS and BPF were shown to increase PPARγ mRNA in the range of 1–25 µM and 10–25 µM for BPS and BPF, respectively, and promote lipid accumulation and adipogenesis (Reina-Pérez et al. 2021). It has been well documented that TBBPA and its derivatives are able to increase PPARγ mRNA expression in the 3T3-L1 cell line (Riu et al. 2011). Moreover, Wójtowicz et al. (2014) reported that TBBPA acted through the PPARγ pathway in primary mouse neocortical neurons and observed decreased levels of these receptors (Wojtowicz et al. 2014). Therefore, our data are consistent with the current state of knowledge. Moreover, it was demonstrated that 10 and 50 µM TBBPA increased NFE2L2 protein levels in the human hepatoma Hep3B cell line, which, according to these authors, indicated the mechanism of antioxidant enzyme expression control (Oguro et al. 2021). Unfortunately, data concerning NFE2L2 expression after BPS and BPF treatments are not available. It was shown that TBBPA-induced oxidative stress and apoptosis in L02 cells was mediated through the NFE2L2 signaling pathway (Zhang et al. 2019). Interestingly, TBBPA concentrations of 12.5 to 50 µM decreased NFE2L2 protein expression in C17.2 cells (Cho et al. 2020). It was also reported that 10 nM BPA enhanced the NF-κB-IL-6 signaling pathway in the SH-SY5Y cell line and increased its malignancy (Xiong et al. 2017). An increase in NF-κB protein expression was observed also in primary macrophages isolated from red common carp following 100 µg L−1 BPA treatment (Yang et al. 2015). Moreover, BPS and BPF elevated NF-κB mRNA expression at concentration ranges of 100–1000 µg L−1 and 10–1000 µg L−1, respectively (Qiu et al. 2018). Guan et al. (2021) showed that nanomolar concentrations of TBBPA were sufficient to increase NF-κB protein expression in the human Ihikawa endometrial carcinoma cell line. Similarly, in the mouse macrophage RAW 264.7 cell line, TBBPA concentration in the range of 10–50 µM increased NF-κB levels (Han et al. 2009). The involvement of the SHH gene in the mechanism of action of BPA, BPS, BPF, and TBBPA is mainly unknown. The only available data showed that BPA in the human adrenocortical H295A cell line activated the SHH signaling pathway and increased its expression via estrogen receptor beta (Erβ) (Medwid et al. 2018).
Conclusions
Summarizing, presented results clearly show that the tested bisphenol analogs (BPA, BPS, BPF, and TBBPA) are characterized by high negative mechanism of action on microorganisms, plants, and cell proliferation. The observed effect was strict correlated with the genotoxicity, direct impact on metabolism as well as on mRNA expression on genes related to the proliferation, apoptosis, and inflammation in keratinocytes in vitro. Moreover, our results showed high genotoxicity of BPA, TBBPA, BPF, and BPS before and after metabolic activation with rat liver S9 fraction. Furthermore, the tested bisphenols were able to significantly inhibit the growth of the tested microorganisms (bacteria and yeast). The phytotoxicity test showed that bisphenols mainly inhibited root growth, proving the negative impact of such substance on root system. Our study sheds new light on the negative effect of bisphenol compounds on environment, which is of high importance considering their high content in daily care skin products.
Data availability
Original data available for request.
Abbreviations
- ACTB:
-
Beta-actin
- ATM:
-
Ataxia telangiectasia mutated
- β-gal:
-
β-Galactosidase
- BPA:
-
Bisphenol A
- BPF:
-
Bisphenol F
- BPS:
-
Bisphenol S
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- KI67:
-
Antigen Ki-67
- TBBPA:
-
Tetrabromobisphenol A
- CAT:
-
Catalase
- CIF:
-
Corrected induction factor
- GI:
-
Germination index
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- p53:
-
Protein 53
- RGI:
-
Root growth inhibition
- ROS:
-
Reactive oxygen species
- SHH:
-
Sonic hedgehog homolog
- SOD:
-
Superoxide dismutase
- 2AA:
-
2-Aminoanthracene
- 4NQO:
-
4-Nitroquinoline 1-oxide
References
Abdel-Razek AS, Refaat BM, Abdel-Shakour EH et al (2015) Biodegradation of phenol by Microbacterium terregenes isolated from oil field NORM SOIL. J Appl Environ Microbiol. 3:63–69. https://doi.org/10.12691/jaem-3-3-1
Abdel-Tawwab M, Hamed HS (2018) Effect of bisphenol A toxicity on growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus. J Appl Ichthyol 34:1117–1125. https://doi.org/10.1111/jai.13763
Abraham A, Chakraborty P (2020) A review on sources and health impacts of bisphenol A. Rev Environ Health 35:201–210. https://doi.org/10.1515/reveh-2019-0034
Ahmed S, Atlas E (2016) Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes 40:1566–1573. https://doi.org/10.1038/ijo.2016.95
Ali MM, Fatima A, Nawaz S, et al (2022) Cytotoxic and genotoxic evaluation of bisphenol S on onion root tips by Allium cepa and comet tests. Environ Sci Pollut Reshttps://doi.org/10.1007/s11356-022-21888-2
Apaydin FG, Aslanturk A, Uzunhisarcikli M et al (2019) Histopathological and biochemical studies on the effect of curcumin and taurine against bisphenol A toxicity in male rats. Environ Sci Pollut Res 26:12302–12310. https://doi.org/10.1007/s11356-019-04578-4
Bisht S, Pandey P, Sood A et al (2010) Biodegradation of naphthalene and anthracene by chemo-tactically active rhizobacteria of Populus deltoides. Brazilian J Microbiol 41:922–930. https://doi.org/10.1590/S1517-83822010000400011
Bolli A, Galluzzo P, Ascenzi P et al (2008) Laccase treatment impairs bisphenol A-induced cancer cell proliferation affecting estrogen receptor α-dependent rapid signals. IUBMB Life 60:843–852. https://doi.org/10.1002/iub.130
Bousoumah R, Leso V, Iavicoli I et al (2021) Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: a systematic review. Sci Total Environ 783:146905. https://doi.org/10.1016/j.scitotenv.2021.146905
Braña V, Cagide C, Morel MA (2016) The sustainable use of delftia in agriculture, bioremediation, and bioproducts synthesis. In: microbial models: from environmental to industrial sustainability. Springer Singapore, Singapore, pp 227–247. https://doi.org/10.1007/978-981-10-2555-6_11
Caballero-Casero N, Lunar L, Rubio S (2016) Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids. A Review Anal Chim Acta 908:22–53. https://doi.org/10.1016/j.aca.2015.12.034
Cao S, Wang S, Zhao Y et al (2020) Fate of bisphenol S (BPS) and characterization of non-extractable residues in soil: insights into persistence of BPS. Environ Int 143:105908
Careghini A, Mastorgio AF, Saponaro S, Sezenna E (2015) Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res 22:5711–5741. https://doi.org/10.1007/s11356-014-3974-5
Chen F, Ying GG, Kong LX et al (2011) Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ Pollut 159:1490–1498. https://doi.org/10.1016/j.envpol.2011.03.016
Chen D, Kannan K, Tan H et al (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity - a review. Environ Sci Technol 50:5438–5453. https://doi.org/10.1021/acs.est.5b05387
Cho J-H, Lee S, Jeon H et al (2020) Tetrabromobisphenol A-induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction. Neurotox Res 38:74–85. https://doi.org/10.1007/s12640-020-00179-z
Corrales J, Kristofco LA, Baylor Steele W et al (2015) Global assessment of bisphenol a in the environment: review and analysis of its occurrence and bioaccumulation. Dose-Response 13:1–29. https://doi.org/10.1177/1559325815598308
Cosentino S, Aureli F, Iannilli V (2022) Bisphenols A and its analogues induce genotoxic damage in marine and freshwater amphipods. Environ Adv 7:100183. https://doi.org/10.1016/j.envadv.2022.100183
Doerge DR, Twaddle NC, Vanlandingham M et al (2011) Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol 255:261–270. https://doi.org/10.1016/j.taap.2011.07.009
Dogan M, Yumrutas O, Saygideger SD et al (2010) Effects of bisphenol A and tetrabromobisphenol A on chickpea roots in germination stage. J Agric Environ Sci 9:186–192
Dueñas-Moreno J, Mora A, Cervantes-Avilés P, Mahlknecht J (2022) Groundwater contamination pathways of phthalates and bisphenol A: origin, characteristics, transport, and fate – a review. Environ Int 170. https://doi.org/10.1016/j.envint.2022.107550
European Commission (EC) (1993) Directive 93/67 of 20 July 1993, laying down the principles for the assessment of riks to man and the environment of substances notified in accordance with council directive 67/548/EEC. Off J Eur Communities L227:
European Commission Joint Research Center (2008) European Union Risk Assessment Report CAS: 80-05-7 EINECS No: 201-245-8. Environment Addendum
Fernandez MF, Arrebola JP, Taoufiki J et al (2007) Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol 24:259–264. https://doi.org/10.1016/j.reprotox.2007.06.007
Filardi T, Panimolle F, Lenzi A, Morano S (2020) Bisphenol A and phthalates in diet: an emerging link with pregnancy complications. Nutrients 12:525. https://doi.org/10.3390/nu12020525
Gabrielson J, Kühn I, Colque-Navarro P et al (2003) Microplate-based microbial assay for risk assessment and (eco)toxic fingerprinting of chemicals. Anal Chim Acta 485:121–130. https://doi.org/10.1016/S0003-2670(03)00404-5
Gallart-Ayala H, Moyano E, Galceran MT (2010) On-line solid phase extraction fast liquid chromatography-tandem mass spectrometry for the analysis of bisphenol A and its chlorinated derivatives in water samples. J Chromatogr A 1217:3511–3518. https://doi.org/10.1016/j.chroma.2010.03.028
Gao P, Wang L, Yang N et al (2020) Peroxisome proliferator-activated receptor gamma (PPARγ) activation and metabolism disturbance induced by bisphenol A and its replacement analog bisphenol S using in vitro macrophages and in vivo mouse models. Environ Int 134:105328. https://doi.org/10.1016/j.envint.2019.105328
Genuis SJ, Beesoon S, Birkholz D, Lobo RA (2012) Human excretion of bisphenol A: blood, urine, and sweat (BUS) study. J Environ Public Health 2012:1–10. https://doi.org/10.1155/2012/185731
George O, Bryant BK, Chinnasamy R et al (2008) Bisphenol A directly targets tubulin to disrupt spindle organization in embryonic and somatic cells. ACS Chem Biol 3:167–179. https://doi.org/10.1021/cb700210u
Gibson R, Durán-Álvarez JC, Estrada KL et al (2010) Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 81:1437–1445. https://doi.org/10.1016/j.chemosphere.2010.09.006
Grandin FC, Lacroix MZ, Gayrard V et al (2018) Bisphenol S instead of bisphenol A: toxicokinetic investigations in the ovine materno-feto-placental unit. Environ Int 120:584–592. https://doi.org/10.1016/j.envint.2018.08.019
Guan G, Su H, Wei X et al (2021) The promotion of tetrabromobisphenol A exposure on Ishikawa cells proliferation and pivotal role of ubiquitin-mediated IκB′ degradation. Ecotoxicol Environ Saf 207:111254
Han EH, Park JH, Kang KW et al (2009) Risk assessment of tetrabromobisphenol a on cyclooxygenase-2 expression via map kinase/NF-B/AP-1 signaling pathways in murine macrophages. J Toxicol Environ Heal - Part A Curr Issues 72:1431–1438. https://doi.org/10.1080/15287390903212873
Harris JD, Coon CM, Doherty ME et al (2022) Engineering and characterization of dehalogenase enzymes from Delftia acidovorans in bioremediation of perfluorinated compounds. Synth Syst Biotechnol 7:671–676. https://doi.org/10.1016/j.synbio.2022.02.005
He F, Ru X, Wen T (2020) NRF2, a transcription factor for stress response and beyond. Int J Mol Sci 21:4777. https://doi.org/10.3390/ijms21134777
Hercog K, Maisanaba S, Filipič M et al (2019) Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci Total Environ 687:267–276. https://doi.org/10.1016/j.scitotenv.2019.05.486
Holmes R, Ma J, Andra SS, Wang H-S (2021) Effect of common consumer washing methods on bisphenol A release in tritan drinking bottles. Chemosphere 277:130355. https://doi.org/10.1016/j.chemosphere.2021.130355
Honkisz E, Wójtowicz AK (2015) The role of PPARγ in TBBPA-mediated endocrine disrupting effects in human choriocarcinoma JEG-3 cells. Mol Cell Biochem 409:81–91. https://doi.org/10.1007/s11010-015-2514-z
Huang M, Liu S, Fu L et al (2020) Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere 253:126707. https://doi.org/10.1016/j.chemosphere.2020.126707
Hwang JK, Min KH, Choi KH et al (2013) Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sci 93:367–372. https://doi.org/10.1016/j.lfs.2013.07.020
Ivanov DP, Parker TL, Walker DA et al (2014) Multiplexing spheroid volume, resazurin and acid phosphatase viability assays for high-throughput screening of tumour spheroids and stem cell neurospheres. PLoS One 9:e103817. https://doi.org/10.1371/journal.pone.0103817
Jalal N, Surendranath AR, Pathak JL et al (2018) Bisphenol A (BPA) the mighty and the mutagenic. Toxicol Reports 5:76–84. https://doi.org/10.1016/j.toxrep.2017.12.013
Janani C, RanjithaKumari BD (2015) PPAR gamma gene - a review. Diabetes Metab Syndr Clin Res Rev 9:46–50. https://doi.org/10.1016/j.dsx.2014.09.015
Jeong SW, Lee SM, Yum SS et al (2013) Genomic expression responses toward bisphenol-A toxicity in Daphnia magna in terms of reproductive activity. Mol Cell Toxicol 9:149–158. https://doi.org/10.1007/s13273-013-0019-y
Kang J, Katayama Y, Kondo F (2006) Biodegradation or Metabolism of Bisphenol A : from Microorganisms to Mammals 217:81–90. https://doi.org/10.1016/j.tox.2005.10.001
Kim D, Il KJ, An YJ (2018) Effects of bisphenol A in soil on growth, photosynthesis activity, and genistein levels in crop plants (Vigna radiata). Chemosphere 209:875–882. https://doi.org/10.1016/j.chemosphere.2018.06.146
Kousaiti A, Hahladakis JN, Savvilotidou V et al (2020) Assessment of tetrabromobisphenol-A (TBBPA) content in plastic waste recovered from WEEE. J Hazard Mater 390:121641. https://doi.org/10.1016/j.jhazmat.2019.121641
Langdon KA, Warne MSJ, Smernik RJ et al (2012) Chemosphere field dissipation of 4-nonylphenol, 4-t-octylphenol, triclosan and bisphenol A following land application of biosolids. Chemosphere 86:1050–1058. https://doi.org/10.1016/j.chemosphere.2011.11.057
Lee S, Liao C, Song GJ et al (2015) Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 119:1000–1006. https://doi.org/10.1016/j.chemosphere.2014.09.011
Lee J, Cho YS, Jung H, Choi I (2018) Pharmacological regulation of oxidative stress in stem cells. Oxid Med Cell Longev 2018:1–13. https://doi.org/10.1155/2018/4081890
Li X, Wang L, Shen F et al (2018a) Impacts of exogenous pollutant bisphenol A on characteristics of soybeans. Ecotoxicol Environ Saf 157:463–471. https://doi.org/10.1016/j.ecoenv.2018.04.013
Li X, Wang L, Wang S et al (2018b) A preliminary analysis of the effects of bisphenol A on the plant root growth via changes in endogenous plant hormones. Ecotoxicol Environ Saf 150:152–158. https://doi.org/10.1016/j.ecoenv.2017.12.031
Li Y, Liu H, Zhang L et al (2021) Phenols in soils and agricultural products irrigated with reclaimed water. Environ Pollut 276:116690. https://doi.org/10.1016/j.envpol.2021.116690
Liu J, Zhang L, Lu G et al (2021) Occurrence, toxicity and ecological risk of bisphenol A analogues in aquatic environment – a review. Ecotoxicol Environ Saf 208:111481. https://doi.org/10.1016/j.ecoenv.2020.111481
Lloyd V, Morse M, Purakal B et al (2019) Hormone-like effects of bisphenol A on p53 and estrogen receptor alpha in breast cancer cells. Biores Open Access 8:169–184. https://doi.org/10.1089/biores.2018.0048
Lucendo-Villarin B, Nell P, Hellwig B et al (2020) Genome-wide expression changes induced by bisphenol a, f and s in human stem cell derived hepatocyte-like cells. EXCLI J 19:1459–1476. https://doi.org/10.17179/excli2020-2934
Lv T, Shen L, Yang L et al (2018) Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway. Int Immunopharmacol 64:256–263. https://doi.org/10.1016/j.intimp.2018.09.009
Mahemuti L, Chen Q, Coughlan MC et al (2018) Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch Toxicol 92:1453–1469. https://doi.org/10.1007/s00204-017-2150-3
Masoner JR, Kolpin DW, Furlong ET et al (2014) Contaminants of emerging concern in fresh leachate from landfills in the conterminous United States. Environ Sci Process Impacts 16:2335–2354. https://doi.org/10.1039/C4EM00124A
Mazan-Mamczarz K, Hagner PR, Zhang Y et al (2011) ATM regulates a DNA damage response posttranscriptional RNA operon in lymphocytes. Blood 117:2441–2450. https://doi.org/10.1182/blood-2010-09-310987
Medwid S, Guan H, Yang K (2018) Bisphenol A stimulates adrenal cortical cell proliferation via ERβ-mediated activation of the sonic hedgehog signalling pathway. J Steroid Biochem Mol Biol 178:254–262. https://doi.org/10.1016/j.jsbmb.2018.01.004
Melcer H, Klecka G (2011) Treatment of wastewaters containing bisphenol A: state of the science review. Water Environ Res 83:650–666
Miller I, Min M, Yang C et al (2018) Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep 24:1105-1112.e5. https://doi.org/10.1016/j.celrep.2018.06.110
Mishra S, Lin Z, Pang S, et al (2021) Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front Bioeng Biotechnol 9. https://doi.org/10.3389/fbioe.2021.632059
Morales M, de la Fuente M, Martín-Folgar R (2020) BPA and its analogues (BPS and BPF) modify the expression of genes involved in the endocrine pathway and apoptosis and a multi drug resistance gene of the aquatic midge Chironomus riparius (Diptera). Environ Pollut 265:114806. https://doi.org/10.1016/j.envpol.2020.114806
Mustieles V, Fernández MF (2020) Bisphenol A shapes children’s brain and behavior: towards an integrated neurotoxicity assessment including human data. Environ Heal A Glob Access Sci Source 19:1–8. https://doi.org/10.1186/s12940-020-00620-y
Oguro A, Sugitani A, Kobayashi Y et al (2021) Bisphenol a stabilizes Nrf2 via Ca2+ influx by direct activation of the IP3 receptor. J Toxicol Sci 46:1–10. https://doi.org/10.2131/jts.46.1
Park SY, Choi EM, Suh KS et al (2019) Tetrabromobisphenol a promotes the osteoclastogenesis of RAW264.7 cells induced by receptor activator of NF-kappa B ligand in vitro. J Korean Med Sci 34:1–12. https://doi.org/10.3346/jkms.2019.34.e267
Pérez RA, Albero B, Férriz M, Tadeo JL (2017) Rapid multiresidue determination of bisphenol analogues in soil with on-line derivatization. Anal Bioanal Chem 409:4571–4580. https://doi.org/10.1007/s00216-017-0399-2
Qiu W, Shao H, Lei P et al (2018) Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 194:1–8. https://doi.org/10.1016/j.chemosphere.2017.11.125
Qiu W, Zhan H, Hu J et al (2019) The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: a critical review of recent progress. Ecotoxicol Environ Saf 173:192–202. https://doi.org/10.1016/j.ecoenv.2019.01.114
Ramos C, Ladeira C, Zeferino S et al (2019) Cytotoxic and genotoxic effects of environmental relevant concentrations of bisphenol A and interactions with doxorubicin. Mutat Res - Genet Toxicol Environ Mutagen 838:28–36. https://doi.org/10.1016/j.mrgentox.2018.11.009
Reale E, Vernez D, Hopf NB (2021) Skin absorption of bisphenol A and its alternatives in thermal paper. Ann Work Expo Heal 65:206–218. https://doi.org/10.1093/annweh/wxaa095
Reina-Pérez I, Olivas-Martínez A, Mustieles V, et al (2021) Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem Toxicol 152. https://doi.org/10.1016/j.fct.2021.112216
Ribeiro-Varandas E, Viegas W, Sofia Pereira H, Delgado M (2013) Bisphenol A at concentrations found in human serum induces aneugenic effects in endothelial cells. Mutat Res - Genet Toxicol Environ Mutagen 751:27–33. https://doi.org/10.1016/j.mrgentox.2012.10.007
Riu A, Grimaldi M, le Maire A et al (2011) Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119:1227–1232. https://doi.org/10.1289/ehp.1003328
Ruan T, Liang D, Song S et al (2015) Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124:150–155. https://doi.org/10.1016/j.chemosphere.2014.12.017
Shan XM, Shen DH, Wang BS et al (2014) Simultaneous determination of bisphenols and alkylphenols in water by solid phase extraction and ultra performance liquid chromatography-tandem mass spectrometry. Biomed Environ Sci 27:471–474. https://doi.org/10.3967/bes2014.076
Singh BK, Walker A, Morgan JAW, Wright DJ (2003) Role of soil pH in the development of enhanced biodegradation of fenamiphos. Appl Environ Microbiol 69:7035–7043. https://doi.org/10.1128/AEM.69.12.7035-7043.2003
Skóra B, Piechowiak T, Szychowski KA (2022) Epidermal growth factor-labeled liposomes as a way to target the toxicity of silver nanoparticles into EGFR-overexpressing cancer cells in vitro. Toxicol Appl Pharmacol 443:116009. https://doi.org/10.1016/j.taap.2022.116009
Staples C, Friederich URS, Hall T et al (2010) Estimating potential risks to terrestrial invertebrates and plants exposed to bisphenol a in soil amended with activated sludge biosolids. Environ Toxicol Chem 29:467–475. https://doi.org/10.1002/etc.49
Szychowski KA, Wójtowicz AK (2013) Components of plastic disrupt the function of the nervous system. Postepy Hig Med Dosw 67:499–506. https://doi.org/10.5604/17322693.1051001
Szychowski KA, Wójtowicz AK (2016) TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacol Reports 68:20–26. https://doi.org/10.1016/j.pharep.2015.06.005
Tian YS, Jin XF, Fu XY et al (2014) Microarray analysis of differentially expressed gene responses to bisphenol A in Arabidopsis. J Toxicol Sci 39:671–679. https://doi.org/10.2131/jts.39.671
Wang S, Wang L, Hua W et al (2015) Effects of bisphenol A, an environmental endocrine disruptor, on the endogenous hormones of plants. Environ Sci Pollut Res 22:17653–17662. https://doi.org/10.1007/s11356-015-4972-y
Wang Q, Chen M, Shan G et al (2017) Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci Total Environ 598:814–820. https://doi.org/10.1016/j.scitotenv.2017.04.167
Wojtowicz AK, Szychowski KA, Kajta M (2014) PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotox Res 25:311–322. https://doi.org/10.1007/s12640-013-9434-z
Wu S, Ji G, Liu J et al (2016) TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ Toxicol 31:1241–1249. https://doi.org/10.1002/tox.22131
Xiao C, Wang L, Zhou Q, Huang X (2020) Hazards of bisphenol A (BPA) exposure: a systematic review of plant toxicology studies. J Hazard Mater 384:121488
Xie H, Wang H, Ji F et al (2018) Tetrabromobisphenol A alters soil microbial community via selective antibacterial activity. Ecotoxicol Environ Saf 164:597–603. https://doi.org/10.1016/j.ecoenv.2018.08.053
Xing J, Zhang S, Zhang M, Hou J (2022) A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp Biochem Physiol Part - C Toxicol Pharmacol 254:109275. https://doi.org/10.1016/j.cbpc.2022.109275
Xiong S, Wang Y, Li H, Zhang X (2017) Low dose of bisphenol A activates NF-κB/IL-6 signals to increase malignancy of neuroblastoma cells. Cell Mol Neurobiol 37:1095–1103. https://doi.org/10.1007/s10571-016-0443-3
Yang Y, Lu L, Zhang J et al (2014) Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 1328:26–34. https://doi.org/10.1016/j.chroma.2013.12.074
Yang M, Qiu W, Chen B et al (2015) The in vitro immune modulatory effect of bisphenol a on fish macrophages via estrogen receptor α and nuclear factor-κB signaling. Environ Sci Technol 49:1888–1895. https://doi.org/10.1021/es505163v
Yin J, Meng Z, Zhu Y et al (2011) Dummy molecularly imprinted polymer for selective screening of trace bisphenols in river water. Anal Methods 3:173–180. https://doi.org/10.1039/c0ay00540a
Zaborowska M, Wyszkowska J, Kucharski J (2019) Biochemical activity of soil contaminated with BPS, bioaugmented with a mould fungi consortium and a bacteria consortium. Environ Sci Pollut Res 26:37054–37069. https://doi.org/10.1007/s11356-019-06875-4
Zaborowska M, Wyszkowska J, Borowik A (2020a) Soil microbiome response to contamination with bisphenol A, bisphenol F and bisphenol S. Int J Mol Sci 21. https://doi.org/10.3390/ijms21103529
Zaborowska M, Wyszkowska J, Kucharski J (2020b) Soil enzyme response to bisphenol F contamination in the soil bioaugmented using bacterial and mould fungal consortium. Environ Monit Assess 192. https://doi.org/10.1007/s10661-019-7999-6
Zaborowska M, Wyszkowska J, Borowik A, Kucharski J (2021) Bisphenol A—a dangerous pollutant distorting the biological properties of soil. Int J Mol Sci 22:12753. https://doi.org/10.3390/ijms222312753
Zhang J, Wang L, Zhou Q, Huang X (2018a) Reactive oxygen species initiate a protective response in plant roots to stress induced by environmental bisphenol A. Ecotoxicol Environ Saf 154:197–205. https://doi.org/10.1016/j.ecoenv.2018.02.020
Zhang YF, Ren XM, Li YY et al (2018b) Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ Pollut 237:1072–1079. https://doi.org/10.1016/j.envpol.2017.11.027
Zhang Y, Wang X, Chen C et al (2019) Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere 226:463–471. https://doi.org/10.1016/j.chemosphere.2019.03.167
Funding
This work was financially supported by the statutory funds of the University of Information Technology and Management in Rzeszow, Poland (DS 503–07-01–27) and the national research internship financed by University of Life Sciences in Lublin, Poland (KSN-w-9/21).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kamila Rybczyńska-Tkaczyk, Bartosz Skóra, and Konrad A. Szychowski. The first draft of the manuscript was written by Kamila Rybczyńska-Tkaczyk. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
We hereby declare that the manuscript is original work by the authors and has not been submitted for publication elsewhere. We further certify that proper citations to the previously reported work have been given and no data/table/figures have been quoted verbatim from other publications without giving due acknowledgments and without the permission of the author(s). Moreover, we confirm that experiments on cell lines do not require the approval of the Bioethics Committee.
Consent to participate
Not applicable.
Consent for publication
The consent of all the authors of this article has been obtained for submitting the article to the Journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• BPA, BPF, BPS, and TBBPA are characterized by bio- and phytotoxicity.
• Molecular mechanism of bisphenol action in HaCaT has been determined.
• Bisphenols show negative effects on HaCaT cells proliferation and genotoxicity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rybczyńska-Tkaczyk, K., Skóra, B. & Szychowski, K.A. Toxicity of bisphenol A (BPA) and its derivatives in divers biological models with the assessment of molecular mechanisms of toxicity. Environ Sci Pollut Res 30, 75126–75140 (2023). https://doi.org/10.1007/s11356-023-27747-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27747-y