Abstract
A novel flower-like CuNiMn-LDH was synthesized and modified, to obtain a promising Fenton-like catalyst, Fe3O4@ZIF-67/CuNiMn-LDH, with a remarkable degradation of Congo red (CR) utilizing H2O2 oxidant. The structural and morphological characteristics of Fe3O4@ZIF-67/CuNiMn-LDH were analyzed via FTIR, XRD, XPS, SEM-EDX, and SEM spectroscopy. In addition, the magnetic property and the surface’s charge were defined via VSM and ZP analysis, respectively. Fenton-like experiments were implemented to investigate the aptness conditions for the Fenton-like degradation of CR; pH medium, catalyst dosage, H2O2 concentration, temperature, and the initial concentration of CR. The catalyst exhibited supreme degradation performance for CR to reach 90.9% within 30 min at pH 5 and 25 °C. Moreover, the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system revealed considerable activity when tested for different dyes since the degradation efficiencies of CV, MG, MB, MR, MO, and CR were 65.86, 70.76, 72.56, 75.54, 85.99, and 90.9%, respectively. Furthermore, the kinetic study elucidated that the CR degradation by the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system obeyed pseudo-first-order kinetic model. More importantly, the concrete results deduced the synergistic effect between the catalyst components, producing a continuous redox cycle consisting of five active metal species. Eventually, the quenching test and the mechanism study proposed the predominance of the radical mechanism pathway on the Fenton-like degradation of CR by the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incessant drain of anthropogenic contaminations causes a significant environmental issue especially organic dyes such as methylene blue, reactive orange, reactive yellow, and Congo red that result from diverse industries like leather, textile, the coloring of pharmaceutical drugs, etc. (Eltaweil et al. 2022, Motawea et al. 2022). Dyes have noxious influences on human well-being even with insignificant concentrations, they may lead to teratogenic, allergenic, carcinogenic, and various disorders in human beings, for example, deficiency in the function of the liver, reproductive system, and kidneys (Basha et al. 2022, Dang et al. 2020, Kassem et al. 2021). Notably, azo dyes exhibit around 50% of the overall dyes manufacturing owing to their broad utilization in industries; in addition, they are the bulkiest group of organic dyes in which their structures contain -N=N- group as a chromophore center (Abd El-Monaem et al. 2023, Gomaa et al. 2022, Oladipo et al. 2019).
Congo red (CR) is a type of anion diazo dye and the most periodically utilized one that could metabolize to the carcinogenic benzidine (Dang et al. 2020). The preponderance of CR dye-containing wastewater induced by printing and dyeing industries causes deleterious impacts on the ecological system (sadek Kadari et al. 2022). Thence, it is vital to treat such noxious effluents using an appropriate treatment manner before drainage into the environment (Hussein et al. 2022, Sundararaman et al. 2018). For this sake, versatile approaches have been fostered to overcome the toxic traces of CR dye in wastewater; membrane filtration, adsorption, and advanced oxidation processes (AOPs) (Guo et al. 2020, Ouyang et al. 2019).
AOPs possess special merits compared with other remediation methods, such as simple operation, promising efficiency, and fewer residuum after the treatment. AOPs involve the degradation of the organic residuals via the reactive oxygen species (ROS) such as sulfate radical (SO4•−) and hydroxyl radical (•OH) to nontoxic compounds or the mineralization of them into CO2 and H2O (Chen et al. 2021). One of the most popular •OH-based AOPs is the Fenton process which has revealed a primer performance in the degradation of several types of persistent dyes like CR (Chu et al. 2020). Such an approach involves the decomposition of hydrogen peroxide (H2O2) via iron-based catalyst under acidic conditions to produce •OH radicals that could attack the targeted organic dye (Azbar et al. 2004, Lu et al. 2022). Interestingly, the Fenton process is more worthwhile than any other AOP technique, owing to the vastly abundant iron sources and their non-toxicity (Duesterberg &Waite 2006, Gallard et al. 1998). In addition, H2O2 is a green potent oxidant, since its utilization produces only oxygen and water without giving rise to the formation of secondary pollutants (Su et al. 2020). Nevertheless, the matrix of iron-based materials needs more improvements to boost the generated electron acceptors or positive charge generators, which enhances the Fe3+/Fe2+ cycle and the generated •OH radicals during the Fenton degradation process (Ouyang et al. 2019).
Layered double hydroxide (LDH) is an anionic clay mineral with superior chemical stability and flexible composition. LDH possesses a special chemical formula, endowing it much interest as a Fenton catalyst; [M2+1-x M3+x(OH)2]x+(An− x/n).mH2O, where M2+ and M3+ are the di and trivalent metal cations, respectively, and An− expresses the anion in the interlayer (Fan et al. 2021, Sobhana et al. 2017). Interestingly, it was reported that the modification of iron materials by LDH prevents the formation of iron sludge; thereby, LDH could ameliorate the materials’ reusability and the usage of H2O2 (Wang et al. 2020). A number of LDHs have been prepared from Cu, Ni, and Mn cations; however, there is no study until now involving the fabrication of CuNiMn-LDH. This novel LDH was expected to be a propitious heterogeneous Fenton-like catalyst in which Cu possesses remarkable catalytic activity owing to its low redox potential (Wu et al. 2022b). In addition, Ni could prevent Cu leaching and facilitate the formation of radical species (Khajeh et al. 2022). Furthermore, the multivalence of Ni and Mn atoms displayed astonishing catalytic activity due to the possibility of electron transfer among them (Zhu et al. 2018).
Metal-organic frameworks (MOFs) are ultra-modern materials possessing striking structural characteristics and various features, like outstanding mechanical/thermal stability, adaptable pore size, available unsaturated coordination spots, considerable specific surface area, etc. (Jin et al. 2022, Zhong et al. 2021). Zeolitic imidazolate framework-67 (ZIF-67) is a subcategory of MOFs that acquire a structure analogous to the ancestor inorganic zeolites, consisting of cobalt ion center and imidazolate organic linkers (Wang et al. 2021, Wu et al. 2022a). ZIF-67 features superior chemical/thermal stability, high pores materials, and abundant active sites (Zhang et al. 2021b). Therefore, it has been broadly adapted in various fields, such as separation, adsorption, and catalysis (Wang et al. 2022, Zhang et al. 2022). ZIF-67 has exhibited a promising performance as a Fenton-like catalyst to degrade many organic contaminants. Importantly, several studies have recommended the combination between ZIF-67 and iron-based materials for an effective Fenton-like degradation process owing to the synergistic effect between Fe and Co metals (Chen et al. 2021, Hashemzadeh et al. 2021).
In light of the aforementioned, we aimed to fabricate a novel CuNiMn-LDH and exploit the features of Fe3O4, and ZIF-67 to enhance its catalytic activity by forming an outstanding heterogeneous Fenton-like catalyst with a continuous redox cycle. It was postulated that the construction of Fe3O4@ZIF-67/CuNiMn-LDH composite will give rise to a stronger synergistic effect between the contained transition metals, thereby, enhancing the degradation of CR. (i) Fe3O4@ZIF-67/CuNiMn-LDH and its pure components were characterized by XRD, ZP, XPS, FTIR, VSM, SEM, and SEM-EDX. (ii) A complete Fenton-like study was proceeded to determine the optimum condition to degrade CR by Fe3O4@ZIF-67/CuNiMn-LDH composite; effect of pH, catalyst dosage, H2O2 concentration, temperature, and initial concentration of CR. (iii) The catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH was evaluated toward different other dyes. (iv) The defining of the radical species and the reaction mechanism were examined by quenching test and XPS of the composite before and after the degradation reaction. (v) The possible degradation pathways of CR by the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system were identified using GC-MS. (vi) The reusability and metal leaching % of Fe3O4@ZIF-67/CuNiMn-LDH were examined.
Experimental section
Materials
The utilized chemicals are listed in Text S1.
Synthesis of Fe3O4
Fe3O4 nanoparticles were synthesized via a superficial reverse co-precipitation approach (Chen et al. 2018). A total of 3.42 g FeSO4.7H2O and 3.33 g FeCl3.6H2O were dissolved in 45 mL distilled water and then transferred into a water bath warmed to 80 °C. For obtaining a homogenous solution, 35.5 μL from concentrated HCl (10 mM) was dipped into the Fe3+/Fe2+ solution, and then stirred for 1 h. Next, NH3.H2O was dropped onto the reaction mixture until pH~10 (Eq. 1). The formed solid was left under stirring for 30 min, then it was collected by utilizing a magnet, washed with ultrapure water, and dried at 60 °C for 12 h.
Synthesis of CuNiMn-LDH
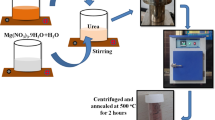
CuNiMn-LDH was synthesized via hydrothermal technique; simply, CuCl2.2H2O, NiCl2.2H2O, and MnCl2.4H2O were dissolved in 60 mL of deionized water with a molar ratio of 1:2:1 and 1:3:2 respectively. Next, 10 mmol of urea was added to the above solution under potent stirring for 60 min at room temperature. The cations/urea solution was transferred into an autoclave, sealed carefully, and warmed to 130 °C for 18 h. Finally, the yielded solid was centrifuged, washed with ultrapure water and alcohol, and finally dried at 60 °C for 10 h.
Synthesis of ZIF-67
A solvothermal approach was applied to prepare ZIF-67 as previously reported by Omer et al. (2021); dissolving Co(NO3)2.6H2O (0.215 g) in 60 mL DMF, then MeIm (0.235 g) was added to the Co2+ and retained them under stirring for 1 h. The Co2+/MeIm solution was poured into a stainless steel autoclave and put in an oven at 125 °C for 24 h. Ultimately, the outcome mulberry solid was double washed with DMF and ethanol and dried for 12 h at 70 °C.
Synthesis of Fe3O4@ZIF-67/CuNiMn-LDH composite
Fe3O4@ZIF-67/CuNiMn-LDH catalyst was fabricated with different mass ratios between ZIF-67: CuNiMn-LDH; 1:1, 1:2, and 2:1, respectively. Simply, 0.01 g Fe3O4 was dispersed in 60 mL DMF followed by mixing a specific amount of CuNiMn-LDH; the mixture was left at room temperature under mild stirring for 30 min for well dispersion. Then, 0.215 g Co(NO3)2.6H2O and 0.235 g MeIm were added and stirred for 1 h. Thereafter, the mixture solution was dipped into a stainless autoclave and heated for 24 h at 125 °C. Finally, the produced magnetic MOF/LDH composite was washed and dried at 70 °C overnight.
Characterization
The synthesized catalysts were analyzed via X-ray diffractometer (XRD- MAC Science M03XHF) to examine the crystalline phase. The chemical composition was identified via Fourier transform-infrared spectra (FTIR- Tensor II, Bruker). Moreover, X-ray photoelectron spectroscopy (XPS- Thermo scientific ESCALAB 250Xi VG) was applied to analyze the samples’ elemental composition, whereas Zeta-sizer (ZP-Malvern) was utilized to examine the surface charge. Also, the samples’ morphologies were analyzed by scanning electron microscope and energy dispersive X-ray microscope (SEM, SEM-EDX- JSM-760F). The magnetization of Fe3O4@ZIF-67/CuNiMn-LDH catalyst was assessed via vibrating sample magnetometer (VSM- Oxford Type 1.2T). The concentrations of the leached metals were determined by an inductively coupled plasma mass spectrometer (ICP-MS, M90, Bruker). The degradation pathways were supposed by Gas chromatography–mass spectrometry (SHIMADZU, GCMS-QP 2010 Ultra).
Fenton-like experiments
Congo red was utilized as a toxic-waste model for evaluating the catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH composite. The degradation experiments are accomplished as follows: under agitation speed 200 rpm, 0.01 g Fe3O4@ZIF-67/CuNiMn-LDH was added to 20 mL CR solution, and 1 mL H2O2. The optimum conditions of the catalytic degradation process were defined by studying the controlling parameters like the solution pH which was scrutinized in the range of 2–10. In addition, the influence catalyst dosage by varying the mass of Fe3O4@ZIF-67/CuNiMn-LDH at the range of 0.005–0.02 g. The influence of the solution temperature was assessed at a temperature ranging from 25 to 55 °C. Also, the impact of the concentration of H2O2 was tested in the range of 100–500 mg/L, and the initial concentration of CR was in the range of 50–200 mg/L. Furthermore, the catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH was assessed toward cationic dyes (MB, MG, and CV) and anionic dyes (MR, MO, and CR). Moreover, the reactive species was defined by quenching test using scavengers such as t-butyl alcohol (TBA) and chloroform. The H2O2 concentration was detected by applying the potassium titanium (IV) oxalate spectrophotometric method with a wavelength adjusted at 385 nm. During the catalytic degradation process, a 3 mL sample of the solution was withdrawn, and its concentration was determined via UV-vis spectrometer at λmax = 500 nm. The CR degradation (%) was calculated from Eq. 2:
Co symbolizes the initial concentration of CR, and Ct expresses the CR concentration at a run time.
Extraction and analysis of CR degradation products
The degradation of CR dye by Fe3O4@ZIF-67/CuNiMn-LDH catalyst produces various intermediates which are identified via GC-MS analysis. After completing the degradation reaction of CR, the degraded sample was centrifuged for 10 min. The produced compounds after the CR degradation were extracted using liquid–liquid extraction by equal ratios of ethyl acetate and samples. The extracted organic phase was dried over Na2SO4, then evaporated till dryness by rotary evaporation. Eventually, the obtained product was dissolved into methanol.
The extracted product was injected into a (0.18 mm diameter × 20 m long, 0.18 mm film thickness) PE-5MS column with initial temperature adjusted at 80 °C for 2 min, then increased by 10 °C min−1 until reaching 280 °C followed by holding for 7 min.
Recycle test
The reusability of AOP catalysts has an immense spotlight in the actual practical application. The reusability of Fe3O4@ZIF-67/CuNiMn-LDH catalyst was estimated by five consecutive runs of CR degradation. After each run, Fe3O4@ZIF-67/CuNiMn-LDH was washed with acetone and dried at 60 °C for 12 h. Then, the regenerated catalyst was examined in the next run.
Results and discussion
Characterization of Fe3O4@ZIF-67/CuNiMn-LDH
FTIR
Figure 1A reveals the FTIR spectra of Fe3O4, ZIF-67, CuNiMn-LDH, and Fe3O4@ZIF-67/CuNiMn-LDH. For Fe3O4, the peaks at 561 and 1417 cm−1 are credited to Fe–O–Fe stretching vibration (Eltaweil et al. 2021). The bands at 892 and 1632 cm−1 are assigned to OH vibration and bending modes, respectively. For ZIF-67, the peaks at 2921 and 1565 cm−1 are ascribed to stretching aromatic ring and stretching C=N of 2-MeIm, sequentially, while the peaks at 1417, 459, and 994 cm−1 are assigned to C=C, Co–N stretching and bending vibration of C–N, respectively (Omer et al. 2021, Wei et al. 2020). For CuNiMn-LDH, the broad band at 3440 cm−1 belongs to O–H stretching, and the absorbance peak at 1630 cm−1 is attributed to the bending vibration of H2O and the interlayer distance of the LDH. Furthermore, the manifestation of the band at 1041 cm−1 is ascribed to the Cu–O, while the peaks ranging from 400 to 700 cm−1 correspond to the bending and stretching modes of M–O bonds and M–OH, where M is credited to Ni and Mn (Baig et al. 2021, Saghir et al. 2020). FTIR elucidates the formation of Fe3O4@ZIF-67/CuNiMn-LDH since the characteristic absorbance bands of Fe3O4, ZIF-67, and CuNiMn-LDH are manifested clearly in the spectrum.
XRD
The crystal profiles of Fe3O4, ZIF-67, CuNiMn-LDH (1:2:1, and 1:3:2), and Fe3O4@ZIF-67/CuNiMn-LDH were inspected by XRD as depicted in Fig. 1B. The XRD profile shows the characteristic diffraction peaks of Fe3O4 at 2θ = 30.1°, 35.8°, 43.1°, 57.01°, and 62.8° which are accompanied by (220), (311), (400), (511), and (440) planes, sequentially (Chen et al. 2021). These characteristic peaks are well consistent with the standard PDF card (JCPDS No. 03-0863). Furthermore, the XRD profile of ZIF-67 depicts sharp peaks at 7.4°, 10.4°, 12.8°, 14.5°, 18.0°, 24.5°, and 26.8° which corresponded to (011), (002), (112), (022), (222), (233), and (134) planes, respectively (Omer et al. 2021). The XRD profile of CuNiMn-LDH (1:2:1) shows a prominent decline in the crystallinity when the Cu ratio elevated in the LDH, owing to the influence of Jahn–Teller distortion, as Cu2+ installed a distorted Cu complex octahedral (Zhu et al. 2022). On the other hand, the XRD pattern of CuNiMn-LDH (1:3:2) elucidated its high crystallinity, revealing the diffraction peaks with high intensity at 11.06°, 22.2°, 32.6°, 38.5°, 47.8°, 59.5°, and 62.9°, corresponding by (003), (006), (101), (015), (018), (110), and (113) planes, respectively. Thence, the ratio of 1:3:2 was chosen as the optimal metal cations ratio to fabricate CuNiMn-LDH. The XRD profile of Fe3O4@ZIF-67/CuNiMn-LDH composite reveals the discriminative diffraction peaks of Fe3O4, ZIF-67, and CuNiMn-LDH peaks, but with lower peaks intensity compared to the pristine phase, emphasizing the successful combination between them.
VSM
VSM hysteresis loops (Fig. 1C) exhibit the soft ferromagnetic nature of Fe3O4 and Fe3O4@ZIF-67/CuNiMn-LDH in which the coercivity value of both magnetic samples exceeded 20 G. Furthermore, it was recorded a noticeable diminution in the saturation magnetization of Fe3O4@ZIF-67/CuNiMn-LDH (12.5 emu/g) compared to the pristine the Fe3O4 (78.5 emu/g). Such a finding is most likely due to the non-magnetic nature of both ZIF-67 and CuNiMn-LDH as well as the low proportion of Fe3O4 in the as-fabricated composite.
XPS
To verify the composition of Fe3O4@ZIF-67/CuNiMn-LDH, XPS analysis was applied. The survey spectrum reveals the main elements of the composite Fe, C, N, Co, O, Cu, Ni, and Mn as exhibited in Fig. 2A. The C1s spectrum (Fig. 2B) points out the peaks at 285.24 and 286.69 eV which can be credited to C–C/C=C and C=N, respectively, and the characteristic peak of the C–N bond appeared at 289.67 eV (Zhang et al. 2021a). The O1s spectrum (Fig. 2C) elucidates a peak at 531.78 eV which corresponds to the surface hydroxyl group, and the peak at 530.48 eV belongs to the M-O bonds (Wu et al. 2022a, Zhao et al. 2018). Furthermore, the peaks of Fe2+ 2p3/2, Fe3+ 2p3/2, Fe2+ 2p1/2, and Fe3+ 2p1/2 are detected nearby 709.70, 712.99, 719.65, and 723.06 eV, respectively (Fig. 2D). The distinguishing peaks of Co 2p3/2; Co2+ (octahedral site), Co2+ (tetrahedral site), and Co3+ (octahedral site) were spotted at 780.03, 781.54, and 786.10 eV, respectively, while the characteristic peaks of Co 2p1/2; Co2+ (octahedral site), Co2+ (tetrahedral site), and Co3+ (octahedral site) appeared at 794.94, 795.96, and 797.10 eV, respectively. Also, there are two peaks at 803.92 and 790.48 eV that are assigned to satellite peaks (Fig. 2E) (Khan et al. 2020, Yu et al. 2018). For the Cu 2p spectrum (Fig. 2F), the peaks at 934.39 and 933.06 eV are assigned to Cu2+ 2p1/2 and Cu+ 2p1/2, respectively, while the peaks at 944.09 and 950.85 eV are ascribed to Cu+ 2p3/2 and Cu2+ 2p3/2, respectively. In addition, the existence of the two satellite peaks is at 940.99 and 954.20 eV (Wang et al. 2018, Wu et al. 2022a). Additionally, the spectrum of Ni 2p illustrates the peaks at 855.86, 873.27, 873.27, and 873.88 eV, which are referred to Ni2+ 2p3/2, Ni3+ 2p1/2, Ni2+ 2p1/2, and Ni3+ 2p1/2, respectively (Fig. 2G) (Yu et al. 2018). For Mn 2p spectrum (Fig. 2H), the Mn 2p3/2 peaks spotted at 642.34 eV, 644.74 eV, and 647.89 eV belong to Mn2+, Mn3+, and Mn4+, respectively (Wu et al. 2022b), while the Mn 2p1/2 peaks located at 652.04 eV and 655.39 eV are characterized to Mn2+ and Mn3+, respectively (Chen et al. 2021). Moreover, the discriminative peak of N-H and N of the imidazole ring at 400.23 and 399.19 eV is illustrated in Fig. 2I (Wei et al. 2020).
SEM
For inspection of the catalysts’ morphology, SEM was used as elucidated in Fig. 3. The SEM of Fe3O4 (Fig. 3A) points out a smooth spherical shape in nano size, forming non-uniform agglomerates. The SEM image (Fig. 3B) reveals an irregular morphology of ZIF-67, while Fig. 3C and D figure out the flower-like of CuNiMn-LDH with porous spheres morphology comprised of stacked ultrathin sheets. Moreover, the SEM image of Fe3O4@ZIF-67/CuNiMn-LDH (Fig. 3E, F) shows some distortion in the flower structure of CuNiMn-LDH, which is most likely due to the random allocation of ZIF-67 on the surface of CuNiMn-LDH, in addition to adherence of Fe3O4 nanoparticles. Also, the SEM image clarifies the existence of Fe3O4/ZIF-67 between the ultrathin sheets of CuNiMn-LDH.
Catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH
Comparison test
To scrutinize the synergistic effect between the pure components of Fe3O4@ZIF-67/CuNiMn-LDH composite, a comparison test was executed between Fe3O4, ZIF-67, CuNiMn-LDH, and Fe3O4@ZIF-67/Cu Ni Mn-LDH composites. As exhibited in Fig. 4A, the degradation percent of CR by H2O2, Fe3O4, ZIF-67, CuNiMn-LDH, and Fe3O4@ZIF-67/Cu Ni Mn-LDH reached 10.41, 29.99, 69.13, 59.68, and 90.90%, respectively. The superior degradation percent of Fe3O4@ZIF-67/CuNiMn-LDH owes to the synergistic effect between Fe, Cu, Co, Ni, and Mn which boosts the redox cycle of Fe3+/Fe2+, Cu2+/Cu+, Co3+/Co2+, Ni2+/Ni3+, and Mn3+/Mn4+ (Wang et al. 2018, Wu et al. 2022a). Furthermore, different ratios of MOF: LDH in the catalyst were investigated; where, the degradation efficiency attained 85.5, 90.9, and 88.9% for MOF: LDH with ratios 1:2, 1:1, and 2:1, respectively. Consequently, the composite which contains the equal ratio between MOF and LDH was chosen for the rest experiments.
A Comparison test between H2O2, Fe3O4, CuNiMn-LDH, ZIF-67, and Fe3O4@ZIF-67/CuNiMn-LDH composites (t = 30 min, H2O2 concentration = 500 mg/L, T = 25 °C, catalyst dosage = 0.01 g, and the CR initial concentration = 50 mg/L), and B the degradation activity of Fe3O4@ZIF-67/CuNiMn-LDH toward different dyes (t= 30 min, H2O2 concentration = 500 mg/L, T = 25 °C, catalyst dosage = 0.01 g, and the dyes initial concentration = 50 mg/L)
Fenton-like degradation of various dyes
For further investigation of the catalytic degradation activity of Fe3O4@ZIF-67/CuNiMn-LDH, it was tested in Fenton-like degradation of various organic dyes, CV, MG, MB, MR, and MO. Furthermore, the degradation efficiency of these dyes was compared to that of CR to evince the selectivity of Fe3O4@ZIF-67/CuNiMn-LDH toward CR (Fig. 4B). The catalytic activity test exhibited that the degradation efficiencies of CV, MG, MB, MR, MO, and CR by the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system were 65.86, 70.76, 72.56, 75.54, 85.99, and 90.90%, respectively. Such results imply the propitious activity of the Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system toward the cationic and anionic dyes, but more selective toward the anionic dyes. Importantly, the chemical structure of these dyes plays a vital role in facilitating the degradation process; CR, MR, and MO are functionalized as azo dyes owing to having chromophore centers (-N=N-), which it be easily attacked by •OH radicals due to the existence of the easy-cleavage π-bond. On the other hand, the degradation reactions of MB, MG, and CV take place on the nitrogen-atoms bond of their inner ring. Since the presence of the electron-donating (-N(CH3)2) group notably increased the electron density of nitrogen-atoms that bond to the benzene ring, thereby, the inner rings of MB, MG, and CV are smoothly broken (Zhao et al. 2018). In light of these results, CR was picked out as a model of anionic dye to evaluate the catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH.
Effect of the controlling parameters on the Fenton-like degradation of CR
The influence of pH
Figure 5A illustrated that the catalytic activity of Fe3O4@ZIF-67/CuNiMn-LDH is mainly affected by altering the initial pH value of the CR solution at a pH ranging from 3 to 11. It was deduced that the maximum degradation efficiency of CR by Fe3O4@ZIF-67/CuNiMn-LDH was 90.9% at pH= 5 within 30 min. The results depict that the CR degradation under weak acidic or near neutral conditions was much better than in strongly acidic conditions (pH= 3) or strong basic conditions (pH= 11). At strongly acidic conditions, the produced •OH radicals may be scavenged via H+ causing a striking decrease in the degradation efficiency of CR (Eqs. 3, 4). While the remarkable diminution in the degradation efficiency of CR under strongly basic conditions can be ascribed to the formation of the highly nucleophilic hydroperoxy anions (OOH−) that possesses higher affinity towards metal ions (M= Co, Fe, Cu, Ni, and Mn) than H2O2 (Eqs. 5, 6) (Shen et al. 2017), in addition to the possibility of auto-decomposition of H2O2 in the strongly basic medium as clarified in Eq. 7 (Shi et al. 2018). On the other hand, ZP measurements (Fig. 5B) investigated that the pHzpc of Fe3O4@ZIF-67/CuNiMn-LDH was ~5.8. Thence, the surface of Fe3O4@ZIF-67/CuNiMn-LDH charges with positive charges when pH < 5.8, which enhances the degradation efficiency owing to the electrostatic attraction between the anionic CR and Fe3O4@ZIF-67/CuNiMn-LDH. While at pH > 5.8, the electrostatic repulsion forces between CR and the negatively charged surface of Fe3O4@ZIF-67/CuNiMn-LDH dwindle the CR degradation efficiency.
The influence of catalyst dosage
Figure 6A represents the impact of varying the Fe3O4@ZIF-67/CuNiMn-LDH dosages on the CR degradation efficacy. It is obvious that the raising in Fe3O4@ZIF-67/CuNiMn-LDH dosage from 0.005 to 0.02 g boosts the CR degradation efficiency from 56.5 to 99.6%, which is most likely due to the generation of more free radicals in the presence of excess amounts of the catalyst. However, 0.01 g was chosen as the optimal dosage of Fe3O4@ZIF-67/CuNiMn-LDH for economic and environmental reasons.
The controlling factors on the Fenton-like degradation of CR; A the catalyst dosage (pH= 5, T= 25 °C, H2O2 concentration = 500 mg/L, and CR concentration= 50 mg/L), B the H2O2 concentration (pH=5, catalyst dosage= 0.01 g, CR concentration= 50 mg/L, and T= 25 °C), C the temperature (pH=5, H2O2 concentration = 500 mg/L, catalyst dosage= 0.01 g, and CR initial concentration= 50 mg/L), and D the CR initial concentration (T= 25 °C, pH=5, H2O2 concentration = 500 mg/L, and catalyst dosage= 0.01 g)
The influence of H2O2 concentration
The H2O2 concentration is one of the controlling parameters of Fenton-like reaction since H2O2 is the main source of •OH radicals. Figure 6B exhibits the substantial impact of the H2O2 concentration on the CR degradation aptitude. It was observed that the CR degradation efficiency notably improved from 67.9 to 90.9% by raising the H2O2 concentration from 100 to 500 mg/L, respectively, owing to the increase in the generated •OH radicals. Nevertheless, the further increase in the H2O2 concentration over 500 mg/L declines the CR degradation efficiency since the much more H2O2 concentrations may act as scavengers (Eqs. 8, 9), agreeing with Xin et al. study (Xin et al. 2021).
The influence of temperature
The impact of the reaction temperature on the CR degradation by Fe3O4@ZIF-67/CuNiMn-LDH/H2O2 system was scrutinized as represented in Fig. 6C. The experimental results point out that the increase in the temperature from 25 to 55 °C increases the CR degradation percentage reaching ~100%. This thermal behavior is most probably due to the prompting of the interaction between Fe3O4@ZIF-67/CuNiMn-LDH and H2O2 at higher temperatures, producing more reactive •OH species, which directly enhances the efficiency of the CR degradation process (Luo et al. 2020).
The influence of the initial CR concentration
Figure 6D displays a reduction in the CR degradation efficiency by the Fe3O4@ZIF-67/CuNiMn-LDH/ H2O2 system with increasing the initial concentration of CR. For the initial CR concentrations of 50, 100, 200, and 300 mg/L, the degradation efficiency within 120 min attained 98.8, 89.4, 73.8, and 66.9%, respectively. This decline in the CR degradation percent can be explained by the insufficient accessible active sites on the Fe3O4@ZIF-67/CuNiMn-LDH surface for such high concentrations of CR (Wu et al. 2022a). Besides, the blocking of the active sites by the high concentration of the adsorbed CR leads to fewer production of •OH radicals (Hassani et al. 2018).
Kinetic study
For investigating the CR degradation kinetics, the experimental data were inspected by pseudo-first-order kinetic model (Eq. 10).
Co and C are the initial concentration and the concentration at a certain time of the CR dye, respectively, k (min−1) is the rate constant, and t is reaction time (min).
Figure 7A represents a plot of -ln (C/Co) against time that was applied at different CR concentrations of 50, 100, 200, and 300 mg/L. Such a linear graph and the calculated R2 inferred that the degradation of CR at these concentrations fitted pseudo-first-order kinetic model. The R2 and k values are summarized in Table S1.
Identification of reactive species
For detecting the controlling ROS on the Fenton-like degradation of CR, a quenching test was implemented with TBA and chloroform as scavengers for •OH and •O2− species, respectively [59]. It was deduced that the CR degradation efficiency significantly dwindled from 90.9 to 46.711% in the existence of TBA, while there was no significant decrease in degradation rate when chloroform was added (Fig. 7B). This finding infers the predominance of •OH radical in the CR degradation process over Fe3O4@ZIF-67/CuNiMn-LDH catalyst. On the contrary, •O2− species have an ineffective role in the degradation process.
Degradation mechanism
In general, the activation of H2O2 occurs via two pathways; the first one is the surface oxygen vacancy pathway that takes place via the surface reaction of the oxygen vacancies (OV) on the oxide surface, producing O2 species (Eqs. 11, 12), while the radical reaction is the second pathway for the H2O2 activation via the reaction with metal ion species that generate reactive oxygen radicals (Costa et al. 2006).
Based on the results of the quenching test, it was deduced that the Fenton-like degradation of CR over Fe3O4@ZIF-67/CuNiMn-LDH catalyst proceeds via the radical reaction pathway since the presence of radical scavengers reduced the degradation efficiency.
To propose the CR degradation mechanism via the H2O2 activation by Fe3O4@ZIF-67/CuNiMn-LDH, the XPS spectra of the catalyst before and after the degradation reaction were thoroughly inspected. The Fe 2p spectra of Fe3O4@ZIF-67/CuNiMn-LDH catalyst (Fig. 8A) reveal a diminution in the ratio of Fe3+/Fe2+ from 1.164 to 0.641 after the degradation reaction, accompanied by a slight shift in Fe 2p3/2 from 709.70 eV and 712.99 eV to 710.15 eV and 713.04 eV, respectively, inferring the involvement of Fe species in the catalytic reaction where Fe2+ activates H2O2 following Haber–Weiss mechanism (Eqs. 13, 14) (Zhang et al. 2021b). Furthermore, the ability of Cu+ to cleave –N=N– of azo dyes by Sandmeyer reaction results in the direct degradation of azo dyes to carbon radicals (Eq. 15) (Shen et al. 2017). Figure 8B exhibits a slight shifting in the related peaks to Cu 2p after the CR degradation with a decline in the Cu2+/Cu+ ratio from 1.43 to 0.928, suggesting the participation of Cu+ in the H2O2 activation (Eq. 16). It was proposed that Cu+ could be regenerated via the reaction of Cu2+ with Ni2+ (Eq. 17) since the metal-oxo-metal bridge allows the electron transfer from Ni2+ to Cu2+ (Wang et al. 2018). Notably, the redox potential of Fe3+/Fe2+ (0.77 V) is higher than that of Cu2+/Cu+ (0.15 V), thereby the reduction of Fe3+ by Cu+ to regenerate Fe2+ was thermodynamically more preferable as elucidated in Eq. 18 (Luo et al. 2020). The Co 2p spectra (Fig. 8C) point out a diminution in the ratio of Co3+/Co2+ from 0.289 to 0.224 with a slight shift in Co 2p3/2 peaks since Co2+ and Co3+ shifted from 780.03 eV and 786.10 eV to 781.74 eV and 784.86 eV, respectively, suggesting the contribution of Co2+ to the activation of H2O2 as clarified in Eq. 19. In addition to the shifting in the belonging peaks to Co 2p1/2 in which Co2+ octahedral and Co2+ tetrahedral shifted from 794.94 eV and 795.96 eV to 797.31 eV and 794.98 eV, respectively. Interestingly, the reduction of Co3+ via Fe2+ and Cu+ was predicted since Co3+/Co2+ redox potential (1.808 V) is significantly higher than Fe3+/Fe2+ and Cu2+/Cu+; therefore, Co2+ is easily recovered by Fe2+ and Cu+ (Eqs. 20, 21). The Mn 2p spectra (Fig. 8D) figure out that the ratio of Mn3+/Mn2+ elevated from 1.05 to 1.37, verifying that a high amount of Mn2+ converted to Mn3+ during the CR degradation (Eq. 22). Furthermore, it was noticed that Mn4+ 2p3/2 slightly shifted from 647.89 eV to 655.42 eV with a decline in the atomic percent from 18.40 to 12.01% implies the reduction of Mn4+ to Mn3+, agreeing with Zhao et al. study (Zhao et al. 2018).
Notably, the formed redox cycle in Fe3O4@ZIF-67/CuNiMn-LDH is continuous since the metal species could regenerate each other based on their redox potentials. In addition, H2O2 could be further decomposed by •OH radicals as elucidated in Eq. 23 and generates the unstable hydroperoxyl radical that assists in the recovery M2+ (Eq. 24) (Šuligoj et al. 2020).
On the other hand, the CR adsorption mechanism onto Fe3O4@ZIF-67/CuNiMn-LDH proceeded via the coordination bonds between the transition metal-containing catalyst and the sulfate and amine groups of CR. This suggestion was confirmed throughout the shifting of the XPS peaks of the metals. In addition to the electrostatic interactions between the positively charged Fe3O4@ZIF-67/CuNiMn-LDH (pHzpc ~5.8) and the anionic CR molecules could contribute to the adsorption mechanism of CR. Furthermore, the oxygenated groups on the Fe3O4@ZIF-67/CuNiMn-LDH surface could interact with the hydrogen atoms on CR molecules via H-bonding. In addition, the H-bonds could be formed between hydrogen atoms of Fe3O4@ZIF-67/CuNiMn-LDH and sulfate groups of CR.
In one word, Fe3O4@ZIF-67/CuNiMn-LDH catalyst possesses a wide redox cycle consisting of Fe2+/Fe3+, Co2+/Co3+, Ni2+/Ni3+, Mn2+/Mn3+, and Cu+/Cu2+ (Fig. 9) that provides electrons-rich degradation medium, facilitating the H2O2 activation and producing abundant •OH, thus could degrade CR with a propitious efficiency (Eq. 25).
The degradation pathway of CR
The formed intermediates during the CR degradation were determined via GC-MS analysis (Fig. S1), where the degradation of CR may occur through various steps: (a) cleavage of the C–S bond between the sulfonate groups and aromatic rings, (b) ring opening (cleavage of benzene ring), (c) breaking of azo bonds N=N, (d) cleavage of C–C and C–N bonds (Thomas et al. 2016). Nevertheless, it is impossible to identify all the intermediates of the degradation process as it is considered one of the GC-MS analysis limitations (Aghdasinia et al. 2016). Based on the GC-MS results, the CR degradation underwent asymmetric cleavage; initially, the two sulphonate groups of the CR dye were cleaved by •OH, which was accompanied by withdrawing electrons from naphthalene rings, so ring opening was more favorable for naphthalene rings. The ring opening of benzene and naphthalene ring is done at ortho-position of •OH groups resulting in obtaining diisooctyl phthalate (RT=23.27), which further degraded into dibutyl phthalate (RT=18.03). Then, the further oxidizing by •OH generated benzene dicarboxylic acid (RT=27.66), n-hexadecanoic acid (RT=17.88), allyl phenol (RT=6.56), and phenylacetic acid (RT=5.02). Another pathway can be proposed through the asymmetric cleavage results in two molecules of sodium (4-amino-3-diazenyl naphthalene)-1-sulfonate and p-dihydroxy biphenyl. The latter compound can degrade to benzene propanoic acid (RT=24.90), which with further oxidation, produces phenol and 2-propenyl benzene (RT=6.30). In this investigation, it was noticed that asymmetric cleavage steps did not acquire any amino groups and overcome the production of naphthylamine and benzidine, which are tremendously toxic to the environment.
The kinetic of H2O2 decomposition
Catalysts encompassing transition metals are potent catalysts for the decomposition of H2O2, where the catalyst donates electrons to accelerate the generation of radicals from H2O2 (Tatarchuk et al. 2020). Figure 10A elucidates the swift decomposition of H2O2 over Fe3O4@ZIF-67/CuNiMn-LDH catalyst in the absence of any organic pollutants. Before quantification via spectrophotometry, samples were withdrawn at each specified interval time and filtered. After 180 min, the decomposition efficiency of H2O2 reached 98.3%.
Figure 10B represented a plot between the decomposition degree and time, which indicates the decomposition reaction of H2O2 over Fe3O4@ZIF-67/CuNiMn-LDH catalyst fits first-order reaction kinetics. Therefore, the rate constant value was evaluated from the following equation.
Where [H2O2]t and [H2O2]o are the hydrogen peroxides concentrations at time t and its initial concentration, respectively, and kobsd is the first-order observed rate constant (Maharjan et al. 2020).
Synergistic effect between adsorption and Fenton-like reaction
The adsorption role of Fe3O4@ZIF-67/CuNiMn-LDH in the degradation of CR was evaluated as follows: 10 mg of Fe3O4@ZIF-67/CuNiMn-LDH was added into 20 mL aqueous solution of CR with a concentration 50 mg/L at pH 5. A sample was withdrawn every 5 min, and measured its absorbance intensity via a UV-vis spectrometer. After 30 min, the adsorption of CR onto Fe3O4@ZIF-67/CuNiMn-LDH reached equilibrium, followed by adding 1 mL of H2O2 to initiate Fenton-like reaction. As shown in Fig. 11A, the adsorption % of CR onto Fe3O4@ZIF-67/CuNiMn-LDH slightly increased until attained its maximum percentage (37.77%) after 30 min. Interestingly, after starting Fenton-like reaction, the removal% of CR sharply increased to reach 90.9% within 30 min. This finding suggested the synergistic effect between adsorption and Fenton-like reaction to degrade CR molecules since the adsorbed CR around the active sites of the composite can be attacked effortlessly by the engendered ROS after the H2O2 addition (Lin et al. 2022).
Recycle test
Figure 11B exhibits the degradation efficacy of the reused Fe3O4@ZIF-67/CuNiMn-LDH catalyst over five cycles, denoting the high stability of the catalyst since its catalytic activity towards the CR degradation is still high (75.9%). This diminution in the degradation efficacy can be assigned to the blocking of some active sites with the CR molecules during each cycle and also the possibility of losing some particles during the collection and washing stages.
Metal leaching detection
For investigating the metal leaching from Fe3O4@ZIF-67/CuNiMn-LDH during the Fenton-like degradation of CR, the catalyst was analyzed before and after the degradation reaction by SEM-EDX. Figure 12A elucidates that Fe3O4@ZIF-67/CuNiMn-LDH consists of C, N, O, Mn, Fe, Co, Ni, and Cu with atomic % of 16.94, 2.01, 57.30, 1.53, 1.75, 10.99, 7.64, and 1.84%, respectively, while the EDX pattern of the catalyst after the Fenton-like degradation of CR (Fig. 12B) manifested the existence of S with an atomic % of about 0.38%. In addition, it was observed a slight metal leaching % of Mn, Fe, Co, Ni, and Cu of about 5.88, 12.57, 14.28, 6.28, and 4.89%, respectively.
Then, the concentrations of the leached metals were detected by ICP-MS to evince environmental friendliness, as well as assure the stability of Fe3O4@ZIF-67/CuNiMn-LDH. It was recorded that the leached concentrations of Mn, Fe, Co, Ni, and Cu were 0.083, 0.134, 0.159, 0.095, and 0.080 mg/L, which are lower than the legal limits. Notably, Fe3O4@ZIF-67/CuNiMn-LDH revealed higher durability compared to other relevant catalysts (Hou et al. 2019, Xu et al. 2016). This result confirmed the successful formation of continuous redox cycles between the contained transition metals in Fe3O4@ZIF-67/CuNiMn-LDH with extremely high ability to recover each other.
Comparison study
Table 1 summarizes a comparative study between the degradation performance of Fe3O4@ZIF-67/CuNiMn-LDH and other reported catalysts in previous studies towards CR in different Fenton-like reaction conditions. Interestingly, Fe3O4@ZIF-67/CuNiMn-LDH exhibited superior catalytic activity toward CR (90.9%) within a shorter time and at room temperature. This propitious catalytic performance of our novel composite is most probably due to its unique morphology and synergistic effect between its components that form a continuous redox cycle. Although some catalysts revealed higher catalytic activity toward CR than Fe3O4@ZIF-67/CuNiMn-LDH, they required more complex degradation conditions and higher reaction time. For instance, the CeO2 nanorods catalyst reported by Wei et al. required higher H2O2 concentration and a long reaction time reached 2 h, in addition to Chen et al. deduced that the CR degradation by 4A-Fe@Cu catalyst took 12 h.
Conclusions
In summary, a novel CuNiMn-LDH was synthesized and modified by Fe3O4 and ZIF-67, forming a remarkable heterogeneous Fenton-like Fe3O4@ZIF-67/CuNiMn-LDH catalyst that revealed a synergistic effect between catalytic activity of the components for superb degradation of CR. The acquired results from FTIR, XRD, XPS, SEM-EDX, and SEM studies emphasized the successful preparation of Fe3O4@ZIF-67/CuNiMn-LDH. Furthermore, VSM revealed the soft ferromagnetic nature of Fe3O4@ZIF-67/CuNiMn-LDH, and ZP defined that its pHzpc was ~5.8. Significantly, the CR degradation accomplished 90.9% by the Fe3O4@ZIF-67/Cu Ni Mn-LDH/H2O2 system within 30 min with optimum conditions (pH= 5, catalyst dosage= 0.01, H2O2 concentration= 500 mg/L, temperature= 25 °C, and CR concentration = 50 mg/L). Owing to the synergistic effect between Fe, Cu, Co, Ni, and Mn, Fe3O4@ZIF-67/CuNiMn-LDH catalyst exhibited excellent catalytic activity, in addition to its superb catalytic activity towards other cationic and anionic dyes. Interestingly, it was concluded from the quenching test that the CR degradation by the Fe3O4@ZIF-67/Cu Ni Mn-LDH/H2O2 system proceeded via the radical pathway. This finding was evinced by the mechanism study based on XPS analysis that confirmed the formation of a continuous redox cycle, generating abundant electrons for the H2O2 activation and the •OH production.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abd El-Monaem EM, Ayoup MS, Omer AM, Hammad EN, Eltaweil AS (2023) Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl Water Sci 13:67
Aftab TB, Hussain A, Li D (2019) Application of a novel bimetallic hydrogel based on iron and cobalt for the synergistic catalytic degradation of Congo red dye. J Chin Chem Soc 66:919–927
Aghdasinia H, Bagheri R, Vahid B, Khataee A (2016) Central composite design optimization of pilot plant fluidized-bed heterogeneous Fenton process for degradation of an azo dye. Environ Technol 37:2703–2712
Azbar N, Yonar T, Kestioglu K (2004) Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 55:35–43
Baig MM, Gul IH, Ahmad R, Baig SM, Khan MZ, Iqbal N (2021) One-step sonochemical synthesis of NiMn-LDH for supercapacitors and overall water splitting. J Mater Sci 56:18636–18649
Basha IK, El-Monaem A, Eman M, Khalifa RE, Omer AM, Eltaweil AS (2022) Sulfonated graphene oxide impregnated cellulose acetate floated beads for adsorption of methylene blue dye: optimization using response surface methodology. Sci Rep 12:1–17
Chen H, Wang S, Huang L, Zhang L, Han J, Ren W, Pan J, Li J (2022) Core-shell hierarchical Fe/Cu bimetallic Fenton catalyst with improved adsorption and catalytic performance for Congo red degradation. Catalysts 12:1363
Chen M, Wang N, Wang X, Zhou Y, Zhu L (2021) Enhanced degradation of tetrabromobisphenol A by magnetic Fe3O4@ ZIF-67 composites as a heterogeneous Fenton-like catalyst. Chem Eng J 413:127539
Chen Y, Jin X, Guo P (2018) Preparation of Fe3O4/BiPO4 magnetic nanocomposite and its photocatalytic performance. J Mol Struct 1171:140–149
Chu J-H, Kang J-K, Park S-J, Lee C-G (2020) Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J Water Process Eng 37:101455
Costa RC, Lelis M, Oliveira L, Fabris J, Ardisson JD, Rios R, Silva C, Lago R (2006) Novel active heterogeneous Fenton system based on Fe3− xMxO4 (Fe, Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J Hazard Mater 129:171–178
Dang GH, Le TT, Ta AK, Ho TN, Pham TV, Doan TV, Luong TH (2020) Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide. Green Process Synth 9:567–577
Duesterberg CK, Waite TD (2006) Process optimization of Fenton oxidation using kinetic modeling. Environ Sci Technol 40:4189–4195
Eltaweil AS, El-Monaem EMA, Mohy-Eldin MS, Omer AM (2021) Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci Rep 11:1–15
Eltaweil AS, El-Monaem A, Eman M, El-Subruiti GM, Ali BM, El-Latif A, Mona M, Omer AM (2022) Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. J Porous Mater:1–12
Enlei Z, Jiaoyi W, Guosheng W, Bengui Z, Yingpeng X (2016) Efficient Fenton oxidation of Congo red dye by magnetic MgFe2O4 nanorods. J Nanosci Nanotechnol 16:4727–4732
Fan X, Cao Q, Meng F, Song B, Bai Z, Zhao Y, Chen D, Zhou Y, Song M (2021) A Fenton-like system of biochar loading Fe–Al layered double hydroxides (FeAl-LDH@ BC)/H2O2 for phenol removal. Chemosphere 266:128992
Gallard H, de Laat J, Legube B (1998) Effect of pH on the oxidation rate of organic compounds by Fe {sup II}/H {sub 2} O {sub 2}. Mechanisms and simulation; Influence du pH sur la vitesse doxydation de composes organiques par Fe {sup II}/H {sub 2} O {sub 2}. Mecanismes reactionnels et modelisation. New J Chem 22
Gomaa H, El-Monaem A, Eman M, Eltaweil AS, Omer AM (2022) Efficient removal of noxious methylene blue and crystal violet dyes at neutral conditions by reusable montmorillonite/NiFe2O4@ amine-functionalized chitosan composite. Sci Rep 12:1–16
Guo K, Zheng S, Zhang X, Zhao L, Ji S, Chen C, Wu Z, Wang D, Fang J (2020) Roles of bromine radicals and hydroxyl radicals in the degradation of micropollutants by the UV/bromine process. Environ Sci Technol 54:6415–6426
Hashemzadeh B, Alamgholiloo H, Pesyan NN, Asgari E, Sheikhmohammadi A, Yeganeh J, Hashemzadeh H (2021) Degradation of ciprofloxacin using hematite/MOF nanocomposite as a heterogeneous Fenton-like catalyst: a comparison of composite and core− shell structures. Chemosphere 281:130970
Hassani A, Karaca M, Karaca S, Khataee A, Açışlı Ö, Yılmaz B (2018) Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J Environ Manag 211:53–62
Hazarika KK, Talukdar H, Sudarsanam P, Bhargava SK, Bharali P (2020) Highly dispersed Mn2O3− Co3O4 nanostructures on carbon matrix as heterogeneous Fenton-like catalyst. Appl Organomet Chem 34:e5512
Hou L, Li X, Yang Q, Chen F, Wang S, Ma Y, Wu Y, Zhu X, Huang X, Wang D (2019) Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: performance and mechanism for organic pollutant degradation. Sci Total Environ 663:453–464
Hussein MA, Motawea MM, Elsenety MM, El-Bahy SM, Gomaa H (2022) Mesoporous spongy Ni–Co oxides@ wheat straw-derived SiO2 for adsorption and photocatalytic degradation of methylene blue pollutants. Appl Nanosci 12:1519–1536
Jin J-C, Wang J, Guo J, Yan M-H, Wang J, Srivastava D, Kumar A, Sakiyama H, Muddassir M, Pan Y (2022): A 3D rare cubane-like tetramer Cu (II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf A Physicochem Eng Asp, 130475
Kassem KO, Hussein MA, Motawea MM, Gomaa H, Alrowaili Z, Ezzeldien M (2021) Design of mesoporous ZnO@ silica fume-derived SiO2 nanocomposite as photocatalyst for efficient crystal violet removal: effective route to recycle industrial waste. J Clean Prod 326:129416
Khajeh M, Oveisi AR, Barkhordar A, Rakhshanipour M, Sargazi-Avval H (2022) Ternary NiCuZr layered double hydroxide@ MIL-101 (Fe)-NH2 metal-organic framework for photocatalytic degradation of methylene blue. J Nanostructure Chem 12:105–115
Khan ZH, Gao M, Qiu W, Islam MS, Song Z (2020) Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 246:125701
Lin R, Li Y, Yong T, Cao W, Wu J, Shen Y (2022) Synergistic effects of oxidation, coagulation and adsorption in the integrated Fenton-based process for wastewater treatment: a review. J Environ Manag 306:114460
Lu J, Yue M, Cui W, Sun C, Liu L (2022) Supramolecular photocatalyst of perylene bisimide decorated with α-Fe2O3: efficient photo-Fenton degradation of organic pollutants. Colloids Surf A Physicochem Eng Asp 655:130222
Luo X, Hu H, Pan Z, Pei F, Qian H, Miao K, Guo S, Wang W, Feng G (2020) Efficient and stable catalysis of hollow Cu9S5 nanospheres in the Fenton-like degradation of organic dyes. J Hazard Mater 396:122735
Maharjan A, Dikshit PK, Gupta A, Kim BS (2020) Catalytic activity of magnetic iron oxide nanoparticles for hydrogen peroxide decomposition: optimization and characterization. J Chem Technol Biotechnol 95:2495–2508
Motawea MM, Hussein MA, Elsenety MM, Ali HM, El-Nasr TAS, Gomaa H (2022) Mesoporous hierarchical ZrO2@ rice straw-derived SiO2 nanocomposite for rapid adsorption and sunlight-driven photocatalytic degradation of methylene blue. J Photochem Photobiol A Chem 426:113758
Nadjia L, Abdelkader E, Naceur B, Boukoussa B, Ahmed B (2017): Spinel Ni 0.6 Zn 0.4 Fe 2 O 4 nano-catalyst: synthesis, characterization and heterogeneous fenton-like degradation of Congo red azo-dye, Euro-Mediterranean Conference for Environmental Integration. Springer, pp. 141-144
Oladipo AA, Ifebajo AO, Gazi M (2019) Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of azo dye via Fenton-like reactions. Appl Catal B Environ 243:243–252
Omer AM, Abd El-Monaem EM, Abd El-Latif MM, El-Subruiti GM, Eltaweil AS (2021) Facile fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr (VI) from aqueous solutions. Carbohydr Polym 265:118084
Ouyang J, Zhao Z, Suib SL, Yang H (2019) Degradation of Congo red dye by a Fe2O3@ CeO2-ZrO2/palygorskite composite catalyst: synergetic effects of Fe2O3. J Colloid Interface Sci 539:135–145
Sadek Kadari A, Khane Y, Ech-Chergui AN, Popa A, Silipas D, Bennabi F, Zoukel A, Akyildiz E, Driss-Khodja K, Amrani B (2022) Growth, properties and photocatalytic degradation of congo red using Gd: ZnO thin films under visible light. Inorg Chem Commun:109626
Saghir S, Fu E, Xiao Z (2020) Synthesis of CoCu-LDH nanosheets derived from zeolitic imidazole framework-67 (ZIF-67) as an efficient adsorbent for azo dye from waste water. Microporous Mesoporous Mater 297:110010
Sharmoukh W, Abdelhamid HN (2023) Fenton-like cerium metal–organic frameworks (Ce-MOFs) for catalytic oxidation of olefins, alcohol, and dyes degradation. J Clust Sci:1–11
Shen Y, Zhou Y, Zhang Z, Xiao K (2017) Cobalt–copper oxalate nanofibers mediated Fenton degradation of Congo red in aqueous solutions. J Ind Eng Chem 52:153–161
Shi X, Tian A, You J, Yang H, Wang Y, Xue X (2018) Degradation of organic dyes by a new heterogeneous Fenton reagent-Fe2GeS4 nanoparticle. J Hazard Mater 353:182–189
Sobhana SL, Zhang X, Kesavan L, Liias P, Fardim P (2017) Layered double hydroxide interfaced stearic acid–cellulose fibres: a new class of super-hydrophobic hybrid materials. Colloids Surf A Physicochem Eng Asp 522:416–424
Su P, Zhou M, Song G, Du X, Lu X (2020) Efficient H2O2 generation and spontaneous OH conversion for in-situ phenol degradation on nitrogen-doped graphene: pyrolysis temperature regulation and catalyst regeneration mechanism. J Hazard Mater 397:122681
Šuligoj A, Ristić A, Dražić G, Pintar A, Logar NZ, Tušar NN (2020) Bimetal Cu-Mn porous silica-supported catalyst for Fenton-like degradation of organic dyes in wastewater at neutral pH. Catal Today 358:270–277
Sundararaman S, Kavitha V, Mathew AJ, Seby SM (2018) Performance analysis of heterogenous catalyst support for the decolourisation of azo dye (Congo red) by advanced oxidation process. Biocatal Agric Biotechnol 15:384–389
Tatarchuk T, Shyichuk A, Trawczyńska I, Yaremiy I, Pędziwiatr AT, Kurzydło P, Bogacz BF, Gargula R (2020) Spinel cobalt (II) ferrite-chromites as catalysts for H2O2 decomposition: synthesis, morphology, cation distribution and antistructure model of active centers formation. Ceram Int 46:27517–27530
Thomas M, Naikoo GA, Sheikh MUD, Bano M, Khan F (2016) Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J Photochem Photobiol A Chem 327:33–43
Wang H, Jing M, Wu Y, Chen W, Ran Y (2018) Effective degradation of phenol via Fenton reaction over CuNiFe layered double hydroxides. J Hazard Mater 353:53–61
Wang H, Zhang Z, Jing M, Tang S, Wu Y, Liu W (2020) Synthesis of CuNiSn LDHs as highly efficient Fenton catalysts for degradation of phenol. Appl Clay Sci 186:105433
Wang X, Cheng B, Zhang L, Yu J, Li Y (2022) Synthesis of MgNiCo LDH hollow structure derived from ZIF-67 as superb adsorbent for Congo red. J Colloid Interface Sci 612:598–607
Wang Z, Wang X, Wang L, Wei Y, Zhao Z, Du K, Chen D, Li X, Zhou C, Liu G (2021) ZIF-67-derived Co@ N-PC anchored on tracheid skeleton from sawdust with micro/nano composite structures for boosted methylene blue degradation. Sep Purif Technol 278:119489
Wei D, Li B, Luo L, Zheng Y, Huang L, Zhang J, Yang Y, Huang H (2020) Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: Indispensable role of reactive oxygen species and redox-active moieties. J Hazard Mater 391:122057
Wei X, Wang Y, Feng Y, Xie X, Li X, Yang S (2019) Different adsorption-degradation behavior of methylene blue and Congo red in nanoceria/H2O2 system under alkaline conditions. Sci Rep 9:1–10
Wu X, Sun D, Ma H, Ma C, Zhang X, Hao J (2022a) Activation of peroxymonosulfate by magnetic CuFe2O4@ ZIF-67 composite catalyst for the study on the degradation of methylene blue. Colloids Surf A Physicochem Eng Asp 637:128278
Wu Z, Y-y G, Xin S, Lu L, Huang Z, Li M, Cui Y, Fu R, Wang S (2022b) CuxNiyCo-LDH nanosheets on graphene oxide: an efficient and stable Fenton-like catalyst for dual-mechanism degradation of tetracycline. Chem Eng J 434:134574
Xin S, Liu G, Ma X, Gong J, Ma B, Yan Q, Chen Q, Ma D, Zhang G, Gao M (2021) High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: economical synthesis, catalytic performance and mechanism. Appl Catal B Environ 280:119386
Xu Y, Ai J, Zhang H (2016) The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J Hazard Mater 309:87–96
Yu S, Zhang Y, Lou G, Wu Y, Zhu X, Chen H, Shen Z, Fu S, Bao B, Wu L (2018) Synthesis of NiMn-LDH nanosheet@ Ni3S2 nanorod hybrid structures for supercapacitor electrode materials with ultrahigh specific capacitance. Sci Rep 8:1–12
Zhang Q, Wang Y, Wang Z, Zhang Z, Wang X, Yang Z (2021a) Active biochar support nano zero-valent iron for efficient removal of U (VI) from sewage water. J Alloys Compd 852:156993
Zhang T, Ma Q, Zhou M, Li C, Sun J, Shi W, Ai S (2021b) Degradation of methylene blue by a heterogeneous Fenton reaction catalyzed by FeCo2O4-NC nanocomposites derived by ZIFs. Powder Technol 383:212–219
Zhang X-W, Lan M-Y, Wang F, Yi X-H, Wang C-C (2022) ZIF-67-based catalysts in persulfate advanced oxidation processes (PS-AOPs) for water remediation. J Environ Chem Eng:107997
Zhao X, Niu C, Zhang L, Guo H, Wen X, Liang C, Zeng G (2018) Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere 204:11–21
Zhong L, Zhou H, Li R, Bian T, Wang S, Yuan A (2021) In situ confinement pyrolysis of ZIF-67 nanocrystals on hollow carbon spheres towards efficient electrocatalysts for oxygen reduction. J Colloid Interface Sci 584:439–448
Zhu J, Liu Q, Liu J, Chen R, Zhang H, Li R, Wang J (2018) Ni–Mn LDH-decorated 3D Fe-inserted and N-doped carbon framework composites for efficient uranium (VI) removal. Environ Sci Nano 5:467–475
Zhu J, Zhu Y, Zhou W (2022) Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: key role of superoxide radicals. J Colloid Interface Sci 610:504–517
Acknowledgements
We want to thank the editor and anonymous reviewers for their valuable comments and suggestions for this paper.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.B.: methodology, validation, formal analysis, and writing — original draft. A.E. and E.A.: conceptualization, resources, writing — review and editing, validation, formal analysis, visualization, and supervision. G.E.: validation and supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
The online version contains supplementary material available at XXX. (DOCX 120 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eltaweil, A.S., Bakr, S.S., Abd El-Monaem, E.M. et al. Magnetic hierarchical flower-like Fe3O4@ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: optimization and mechanism. Environ Sci Pollut Res 30, 75332–75348 (2023). https://doi.org/10.1007/s11356-023-27430-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27430-2