Abstract
The objective of this study was to explore the impact of maternal AT during pregnancy on childhood asthma and wheezing, as well as the potential effect modifiers in this association. A cross-sectional study was implemented from December 2018 to March 2019 in Jinan to investigate the prevalence of childhood asthma and wheezing among aged 18 months to 3 years. Then, we conducted a case-control study based on population to explore the association between prenatal different AT exposure levels and childhood asthma and wheezing. The association was assessed by generalized additive models and logistic regression models, and stratified analyses were performed to explore potential effect modifiers. A total of 12,384 vaccinated children participated in screening for asthma and wheezing, 236 cases were screened, as well as 1445 controls were randomized. After adjusting for the covariates, childhood asthma and wheezing were significantly associated with cold exposure in the first trimester, with OR 1.731 (95% CI: 1.117–2.628), and cold exposure and heat exposure in the third trimester, with ORs 1.610 (95% CI: 1.030–2.473) and 2.039 (95% CI: 1.343–3.048). In the third trimester, enhanced impacts were found among girls, children whose distance of residence was close to the nearest main traffic road, and children whose parents have asthma. The study indicates that exposure to extreme AT during the first and third trimesters could increase the risk of childhood asthma and wheezing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is a complex and heterogeneous chronic inflammatory disease of the airways and also one of the leading prevalent chronic disorders in children around the world (Asher et al. 2004; Xie and Wenzel 2013). Not only does childhood asthma impose a significant financial and public health burden on families, but also has a huge impact on children’s development (Davis 2013; Stevens et al. 2003). In the USA, the prevalence of childhood asthma has steadily increased from 8.7% in 2001 to 9.3% in 2010 (Akinbami et al. 2016). Also, evidence from the International Childhood Asthma and Allergy Study suggests that the prevalence of childhood asthma in New Zealand has increased from 24.6% in 1992 to 30.2% in 2010 (Asher et al. 2008). In China, from September 2009 to August 2010, the third nationwide survey of childhood asthma was conducted in 43 cities, and the overall incidence of asthma in urban children aged 0–14 years has tripled in the past 20 years (National Cooperative Group on Childhood et al. 2013). This evidence reveals that the prevalence of childhood asthma has increased dramatically in many countries around the world over the past few decades. Therefore, there is an urgent need to study risk factors for the development of childhood asthma to provide more effective prevention strategies.
Environmental factors have been widely accepted as critical risk factors for the development of asthma, such as ambient temperature (Buckley and Richardson 2012; Hernandez 2016). On the one hand, low temperatures can cause inflammation and narrowing of the airway (Kaminsky et al. 2000), and high temperatures can enhance the growth of indoor allergens, which may attribute to asthma (Hashimoto et al. 2004). On the other hand, compared to adults, extreme temperatures have a greater influence on children due to their absence of resistance as well as vulnerability to dramatic weather changes (McKie 2013). To date, several studies have investigated correlations between ambient temperature and childhood asthma (D'Amato et al. 2014; Xu et al. 2013a, b). A meta-analysis covering 13 countries concluded that a 1 °C decrease in short-term ambient temperature was associated with a 5.5% increase in the risk of childhood asthma (Cong et al. 2017). A large diurnal temperature range may trigger childhood asthma in Brisbane, Australia (Xu et al. 2013a, b). However, the effect of temperature on childhood asthma and its critical period was inconsistent, which might be due to differences in the age, season, and region of the study. Most previous studies were conducted in Europe, Oceania, the USA, and southern China, whereas Jinan is located in eastern China, with a temperate monsoon climate, which is notably different from the previous research locations.
Many recent studies have shown that the effects of environmental factors on the development of asthma and lung function deficits may begin in the uterus (Buteau et al. 2020; Hsu et al. 2015; Lavigne et al. 2018). Prenatal exposure to harmful environmental factors could harm organ development as well as infancy growth and could increase the risk of respiratory diseases in childhood (Gluckman et al. 2008). A study suggested that prenatal exposure to diurnal temperature variation plays an important role in the development of childhood pneumonia (Zheng et al. 2021). Also, the common cold in children was associated with prenatal exposure to air temperature variation (Lu et al. 2018). These studies indicate that extreme ambient temperature during pregnancy increases the risk of childhood respiratory disease.
In addition, since ambient temperature may not accurately describe the body’s perception of outdoor temperature, Steadman (Steadman 1994) introduced apparent temperature (AT) in 1984, a composite biometeorological index that includes ambient temperature, wind speed, and relative humidity, which was believed to be a more objective indicator of the human body’s thermal experiences than ambient temperature alone. A growing number of scientists have focused on the impact of different AT exposure during pregnancy on children’s health. For example, exposure to a high level of AT has been reported to be associated with spontaneous preterm birth in California (Avalos et al. 2017). Additionally, an attempt to explore the association between exposure to different AT during pregnancy and term low birth weight has been conducted in California (Basu et al. 2018), whereas the relationship between AT, as a potential risk factor that may be a better fit for investigating childhood asthma than ambient temperature, and asthma in children has not been reported. In Cape Town, South Africa, a study on the association between AT and asthma was implemented in adults (Shirinde and Wichmann 2022). At high AT levels, women were more susceptible to PM10 and the 15–64 age group was more susceptible to NO2 and SO2. Although the subjects in this study were adults, it still provides potential possible for the association between AT and childhood asthma.
To our knowledge, the studies concerning the relationship between temperature, one of the most important environmental factors, during pregnancy and the risk of asthma and wheezing in children are still limited. Accordingly, we developed a case-control study based on the population of Jinan, Shandong Province, China, to evaluate the effect of AT during pregnancy and the risk of childhood asthma and wheezing. Importantly, we further explored its critical window and examined whether this association was modified by other factors. Nowadays, the impact of climatic conditions on health is receiving more attention (Abhijith et al. 2017; Lee et al. 2021). The findings of this study will give evidence on the impact of AT on the risk of asthma and wheezing in children, which could assist in the prevention of asthma and wheezing in children in the context of climate change.
Methods
Study design
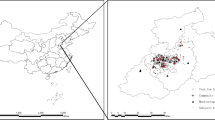
There are two study designs for the present study. Firstly, a cross-sectional study was conducted from December 2018 to March 2019 in Jinan, the capital city of Shandong Province in China (36°40′N, 116°57′E). The 15 community vaccination clinics were selected in five municipal districts of Jinan (Shizhong District, Lixia District, Huaiyin District, Tianqiao District, and Licheng District), as the study sites. The locations of the five municipal districts and the distribution of the clinics were shown in Fig. 1. Children were identified as subjects from the cross-sectional study who attended the 15 community immunization clinics for vaccinations and satisfied the study’s inclusion criteria, and then their mothers completed asthma and wheezing screening. Subsequently, we conducted a case-control study based on the cross-sectional population. Among the participants in the cross-sectional study, children with asthma or wheezing were screened as cases. The purpose and content of the study were explained to each mother, who then signed an informed consent form. The study was allowed by the Ethics Committee of Preventive Medicine of Shandong University (Approved number: 20170315).
Study population and data collection
Inclusion criteria for the subjects were the following: (1) singleton, (2) 18 months to 3 years old, and (3) the mothers who lived in Jinan during pregnancy in the cross-sectional study. Those who had been diagnosed by doctors with wheezing or asthma were defined as cases in the case-control study, which were reported by their mothers. After each case was identified, 1/10 children without asthma and wheezing were randomly selected as controls from vaccination clinics in the National Planned Immunization Registration System. More details, including the method of case and control selection, were presented in another paper (Bai, et al. 2022). Mother-children-pair data were collected by giving out maternal and child health questionnaires to the mothers of the children, which were completed by the children’s mothers under the guidance of uniformly trained investigators. The questionnaire was adapted from the questionnaire of the International Study on Asthma and Allergies in Children (ISAAC) (Asher et al. 2006).
The definition of outcome
In our study, the outcome variable was doctor-diagnosed asthma and wheezing, which was defined by the answer to two questions: (1) Has your child ever been diagnosed with asthma by the doctor? (2) Has your child ever been diagnosed with wheezing by the doctor?
Exposure and the exposure timing windows
Exposure refers to the AT value to which the mother was exposed during pregnancy. We divided the exposure during pregnancy into 4 time windows: the first, second, and third trimesters, and the entire pregnancy, defined as the first 13 weeks of pregnancy, week 14 through 27, week 28 through birth, and the entire gestation period, respectively (Lu et al. 2020).
Exposure assessment of AT and data collection
Daily average meteorological data were obtained from the Shandong Provincial Environmental Information and Monitoring Center, including ambient temperature (° C), relative humidity (%), and wind speed (m/s). The distribution of monitoring stations was relatively concentrated (Fig. 1). The individual exposure level of 3 meteorological factors for exposure timing windows was assessed by an inverse distance weighting (IDW) method based on the subject’s home address code (Bai et al. 2022). The basic idea of IDW is to weigh the level of meteorological factors based on the spatial distance between the specific address and the monitoring station. In particular, exposure levels to meteorological factors are strongly influenced by monitoring concentrations from nearby monitoring stations, but less influenced by monitoring concentrations from remote monitoring stations (Rivera-González et al. 1995). Then, the AT (°C) was calculated from the ambient temperature, relative humidity, and relative wind speed by the following equations (Steadman 1994):
where Tα is the ambient temperature (°C); e is actual vapor pressure (hPa); Rh is relative humidity (%); and Ws is wind speed (m/s). The AT exposure level was divided into three categories, with the extreme heat level being above the 90th percentile (> 90th), which was called heat, the extreme cold level being below the 10th percentile (< 10th), was called cold, and the moderate level being between the 10th and 90th percentile (10th-90th), was called moderate (Gasparrini and Leone 2014).
Covariates
The following variables were collected from the questionnaires as potential covariates: children’s gender (male, female), children’s age, mode of delivery (natural birth, cesarean delivery), preterm birth (no, yes), low birth weight (no, yes), duration of breastfeeding (no, yes), family income monthly, congenital disease (no, yes), maternal age, maternal education, maternal secondhand smoking during pregnancy (no, yes), home dampness (no, yes), the distance of residence from the nearest main traffic road (≤ 200 m, > 200 m), and parental atopy (no, yes). Following that, some covariates were given definitions in the questionnaire. Specifically, preterm birth was defined as delivery before 37 completed weeks of gestation (Vogel et al. 2018). Low birth weight was defined as a birth weight below 2500 g (Hughes et al. 2017). Congenital disease was defined as a structural and functional abnormality of the perinatal infant, which was covered by the birth defects surveillance list and diagnosed by the hospital. Maternal secondhand smoking during pregnancy was defined as the mother’s exposure to tobacco smoke for 30 min or more each week during pregnancy. Home dampness was defined as the presence of at least one of the following in the home: mold, moist spots, damp clothing/bedding, condensation on windows, or musty aromas (Harville and Rabito 2018; Liu et al. 2018). Distance of residence from the nearest main traffic road was defined as the distance between the subject’s residential location and a road having two or more multiple lanes (Miyake et al. 2010). Parental atopy was defined as either the father or mother having been diagnosed with an allergic disease such as asthma and eczema (O'Connor et al. 2022).
Statistical analysis
Continuous variables were described using means, standard deviations (SD), minimum value (Min), maximum value (Max), and percentiles. The Mann-Whitney U test was used to compare the differences in characteristics between the case group and the control group. Categorical variables were expressed in the form of case (percentage) [n (%)], and the Chi-square test or Fisher’s exact test was performed to compare differences among groups. In our study, AT was analyzed as a continuous and categorical variable separately, with the generalized additive model (GAM) for continuous variables and the logistic regression model for categorical variables. Odds ratios (ORs) and 95% of confidence intervals (95% CIs) were calculated to express the effect of different AT exposure during pregnancy on the risk of childhood asthma and wheezing. GAM is a common method to evaluate linear and non-linear correlations, which was used to study the non-linear association between daily average temperature during pregnancy and adverse fetal outcomes (Basagana et al. 2021; Liu et al. 2019). In this study, GAM was used to evaluate the potential non-linear associations between AT during pregnancy and childhood asthma and wheezing, with the number of nodes selected based on the smallest generalized cross-validation (GCV) value. Additionally, the association between different exposure levels of AT (cold, moderate, heat) during pregnancy and childhood asthma and wheezing was evaluated by the logistic regression model. The moderate AT (10th–90th percentile) was set as a reference (OR = 1). Stepwise regression was used to select suitable covariates for the model to control for confounding factors. It is worth noting that previous studies have found that postnatal ambient temperature was also an important factor in asthma attacks in children (Lu et al. 2022). Therefore, we included children’s AT exposure levels from birth to the day of investigation in the model for adjustment. Subsequently, we used stratified analysis to assess the potential effect modification of children’s gender, mode of delivery, maternal vitamin D supplementation during pregnancy, the distance of residence from the nearest main traffic road, and parental atopy. In addition, using the relative excess risk due to interaction (RERI), we studied the interaction between childhood asthma and wheezing and AT as well as these factors. A statistically significant RERI > 0 implies the presence of supra-additivity or synergistic interaction, while a RERI of 0 shows no interaction. Finally, in order to justify the appropriateness of the sample size, statistical power was evaluated using PASS software, according to the method proposed by Hsieh et al. (Hsieh et al. 1998).
Statistical analyses were performed in SPSS (version 24.0, SPSS Inc., Chicago, USA) software, R software (version 4.1.0; www.r-project.org), and PASS software. P < 0.05 was considered statistically significant.
Sensitivity analysis
To examine the stability of the effect of AT during pregnancy on childhood asthma and wheezing that we observed, a sensitivity analysis was implemented in two ways. Firstly, we matched the new control group to cases in the database (1:1), using children’s age and children’s gender as matching criteria. Based on the data of the matched case-control study, we performed a sensitivity analysis. Secondly, the code for community vaccination clinic was set as a dummy variable to investigate whether the subject’s address affects the results. Since immunizations in China have a territorial management mode of 15-min community life cycle, and the vast majority of subjects will go to the nearest community vaccination clinic to be vaccinated, the clinic codes can reflect the different areas of the population.
Results
Descriptive statistics
In the study area, a total of 12,384 children and their mothers filled out asthma and wheezing screening forms with the help of instructors. The prevalence of asthma and wheezing was 2.07% among children aged 18 months to 3 years. Finally, there were 236 cases and 1445 controls in the overall material (N = 1681). Descriptive statistics for characteristics and the prevalence of childhood asthma and wheezing stratified by covariates are shown in Table 1. Children with asthma and wheezing were more frequently born to mothers who were older (≥ 35 years old) and more educated (college or more). Meanwhile, between the two groups, there were differences in children’s age, preterm birth, low birth weight, congenital disease, family income monthly, maternal vitamin D supplementation during pregnancy, home dampness, the distance of residence from the nearest main traffic road, and parental atopy (P < 0.05).
Distribution of AT during pregnancy
The means, SDs, 10th percentile, 90th percentile, maximum, and minimum values of AT exposure level in each time window for the case group and control group are given in Table 2. For the case group, the daily means (SDs) of AT were 13.153 (11.004) °C, 14.902 (10.455) °C, 14.040 (11.321) °C, and 13.590 (3.656) °C in the first, second, third trimesters, and entire pregnancy, respectively. And for the control group, the daily means (SDs) of AT were 14.955 (9.771) °C, 13.890 (11.660) °C, 12.827 (9.828) °C, and 13.429 (3.908) °C, respectively. The mean AT exposure level in the first trimester was significantly lower in the case group than that in the control group, and the difference was statistically significant (P < 0.05).
The comparison of AT exposures level for the case and the control
The AT levels of the case group and control group in each time window are shown in Table 3. The 10th and 90th percentile of AT for all subjects were −0.448 °C and 27.866 °C in the first trimester, −0.695 °C and 28.655 °C in the second trimester, and −0.679 °C and 27.729 °C in the third trimester. The percentages below the 10th AT exposure in the first, second, and third trimesters were 13.5%, 8.5%, and 13.5% in the case group and 9.4%, 10.2%, and 9.4% in the control group, with no statistically significant differences among the two groups (P = 0.128). At the same time, the percentages above the 90th percentile of AT in the three time windows were 11.8%, 7.6%, and 13.1% in the case group and 9.7%, 10.4%, and 9.5% in the control group, with no statistically significant differences among the two groups (P = 0.119). In the entire pregnancy, the 10th and 90th percentiles of AT were 8.877 °C and 18.587 °C, and the percentages below the 10th AT in the two groups were 12.3% and 9.6%, with no statistically significant difference (P = 0.205). Likewise, the percentages above the 90th percentile of AT in the two groups were 7.2% and 10.4%, and the difference was not statistically significant (P = 0.123). In addition, in the first trimester, cold exposure was significantly lower in the control group than in the case group (P = 0.008). Meanwhile, heat exposure was significantly higher in the control group than in the case group in the second trimester (P = 0.002) and the entire pregnancy (P = 0.010).
In addition, AT exposure levels were calculated for the case and control groups from birth to the date of investigation. The daily mean (SD) was 12.168 (0.796) °C in the case group and 11.910 (1.387) °C in the control group. The difference between cases and controls was statistically significant (P = 0.003), and the AT level in case was higher than that in the control.
AT during pregnancy and asthma and wheezing
The potential non-linear associations between AT during pregnancy and childhood asthma and wheezing are provided by the GAM in Fig. 2. In Fig. 2A, when the AT exposure levels in the first trimester were lower than 7.05 °C, the positive association between ATs and the risk of asthma and wheezing was statistically significant. For example, the ORs were 1.689 (95% CI: 1.233–2.312), 1.507 (95% CI: 1.183–1.921), and 1.338 (95% CI: 1.083–1.653) when the AT was − 2 °C, 0 °C, and 2 °C, respectively, with the maximum OR = 1.827 (95% CI: 1.235–2.703) when AT = − 3.42 °C. When AT exposure levels were higher than 26.9 °C, there was also a positive association between ATs and the risk of asthma and wheezing, although it was not statistically significant. The relationship between ATs and the risk of asthma and wheezing showed a statistically significant negative association when ATs were from 11.34 to 22.26 °C, with ORs of 0.755–0.844, with the minimum OR = 0.755 (95% CI: 0.631–0.902, P < 0.000) when AT = 16.63 °C. In Fig. 2C, for the third trimester, the positive association between AT and the risk of asthma and wheezing was statistically significant when AT exposure levels were lower than −0.17 °C and were higher than 24.65 °C. For instance, the ORs were 1.525 (95% CI: 1.100–2.113) and 1.653 (95% CI: 1.194–2.067) when the AT was −2 °C and 28 °C, respectively, with the maximum OR of 1.832 (95% CI: 1.146–2.927) when AT = −3.82 °C and 1.926 (95% CI: 1.208–3.070) when AT = 31.10 °C. Similarly, the relationship between ATs and the risk of asthma and wheezing showed a statistically significant negative association when ATs were from 4.87 to 17.00 °C, with ORs of 0.664–0.810, with the minimum OR = 0.664 (95% CI: 0.537–0.822, P < 0.000) when AT = 10.12 °C. However, we did not find significant effects of AT exposure in the second trimester (Fig. 2B) and entire pregnancy (Fig. 2D) on childhood asthma and wheezing.
Non-linear relationship between AT and childhood asthma and wheezing in each time window. The model was adjusted for children’s age, children’s gender, maternal age, preterm birth, low birth weight, monthly family income, congenital disease, maternal education, maternal secondhand smoking during pregnancy, the distance of residence from the nearest main traffic road, home dampness, parental atopy, and AT exposure levels after birth (from birth to the day of the investigation). A The relationship between AT in the first trimester and childhood asthma and wheezing. B The relationship between AT in the second trimester and childhood asthma and wheezing. C The relationship between AT in the third trimester and childhood asthma and wheezing. D The relationship between AT during the entire pregnancy and childhood asthma and wheezing
Additionally, the association between different exposure levels of AT during pregnancy and childhood asthma and wheezing was presented by the logistic regression model in Fig. 3. In the single-factor model, with reference to the moderate temperature, childhood asthma and wheezing was significantly associated with cold exposure in the first trimester, with OR 1.784 (95% CI: 1.190–2.676), and cold exposure and heat exposure in the third trimester, with ORs 1.584 (95% CI: 1.033–2.430) and 2.303 (95% CI: 1.569–3.381). After adjusting for the covariates, asthma and wheezing in children was significantly related to cold exposure in the first trimester, with OR 1.731 (95% CI: 1.117–2.628). Similarly, this association remained significant in the third trimester, with ORs 1.610 (95% CI: 1.030–2.473) and 2.039 (95% CI: 1.343–3.048).
The ORs and 95% CIs of childhood asthma and wheeze associated with AT in each exposure time window. Definition of abbreviations: cold, extreme AT exposure below the 10th percentile; heat: extreme AT exposure above the 90th percentile. Asterisk (.*) P < 0.05; model was performed without adjustment for the covariates; Model was adjusted for children’s age, children’s gender, maternal age, preterm birth, low birth weight, monthly family income, congenital disease, maternal education, maternal secondhand smoking during pregnancy, the distance of residence from the nearest main traffic road, home dampness, parental atopy, and AT exposure levels after birth (from birth to the day of the investigation)
Effect modification on the association of AT and asthma and wheezing
The results of the stratified analysis for the first, second, and third trimesters, and the entire pregnancy are presented in Fig. 3, which had been adjusted for covariates. The results showed that there were statistically significant effect modifications by children’s gender, mode of delivery, maternal vitamin D supplementation during pregnancy, the distance of residence from the nearest main traffic road, and parental atopy. Especially, in the third trimester, extreme AT exposure was found to have a stronger impact on the development of childhood asthma and wheezing among girls, children whose distance of residence was close to the nearest main traffic road, and children whose parents have asthma.
An interaction analysis on the risk of childhood asthma and wheezing was carried out among different subgroups. For the third trimester, the RERI estimate for the interaction effect of heat exposure and parental atopy on the risk of childhood asthma and wheezing was statistically significant, with RERI 2.625 (95% CI: 0.109–5.141). On the additive scale, during the third trimester, these indicate synergistic effects for interaction (Fig. 4).
Effect of AT on the risk of childhood asthma and wheezing, stratified by covariates, and the joint effects of covariates and AT on the risk of childhood asthma and wheezing, in each exposure time window. Asterisk (*) P < 0.05; model was adjusted for children’s age, children’s gender (except for stratified analyses by infant gender), maternal age, preterm birth, low birth weight, monthly family income, congenital disease, maternal education, maternal secondhand smoking during pregnancy, the distance of residence from the nearest main traffic road (except for stratified analyses by the distance of residence from the nearest main traffic road), home dampness, parental atopy (except for stratified analyses by parental atopy), and AT exposure levels after birth (from birth to the day of the investigation), in four exposure time window
Evaluation of statistical power
The results of statistical power evaluation showed that, with a sample size of 1681 observations, the logistic regression of a binary response variable on an independent variable achieves 0.929 statistical power when the significance level was 0.05.
Results of sensitivity analysis
The result from sensitivity analysis showed no change in the association of AT with childhood asthma and wheezing and based on the data of the matched case-control study, which is shown in Table S1. Meanwhile, we rebuilt the model which included the code for community vaccination clinics in analysis. After adjusting for the code for the community vaccination clinic, the magnitude of the association remained unchanged and was shown in Table S2.
Discussion
In recent years, several studies have explored the relationship between temperature and respiratory diseases in children (Wang 2016; Yamazaki et al. 2015). For instance, a prospective cohort study conducted in China among 2598 children found that prenatal exposure to diurnal temperature variation was significantly associated with the incidence of pneumonia in children aged 3–6 years (Miao et al. 2017). Similarly, Lu et al. carried out a cohort study and discovered that childhood pneumonia was significantly associated with increased exposure to diurnal temperature differences throughout the entire pregnancy (Zheng et al. 2021). Apparently, the results of these studies suggest that extreme AT during pregnancy is associated with the risk of respiratory disease in the offspring. However, studies investigating the relationship between AT during pregnancy and the risk of asthma and wheezing in children are still rare, so more relevant research evidence is necessary. In this study, we conducted a community-based case-control study in Jinan City to investigate the association between maternal exposure to extreme AT during pregnancy and the risk of asthma and wheezing in offspring aged 18 months to 3 years. We found that in the first trimester, the relationship between AT and childhood asthma and wheezing displayed an L-shaped, with low AT exposure associated with a higher risk in comparison to moderate AT exposure. Meanwhile, in the third trimester, the curve seemed to be a definite U-shaped with a bottom, with both low and high AT exposure associated with a higher risk in comparison to moderate AT exposure. After grouping the AT during pregnancy, we found essentially the same trend using a logistic regression model. Our data suggested that exposure to extreme AT during pregnancy can influence the risk of asthma in early childhood. According to the authors’ limited search, this paper may be, to date, the first study to show that maternal AT exposure during pregnancy can influence asthma risk in early childhood. Meanwhile, we observed a non-linear relationship between maternal AT exposure levels during pregnancy and the risk of asthma and wheezing development in offspring.
The relationship between the critical AT exposure window in pregnancy and pregnancy outcome has been reported (Basu et al. 2016), so we wondered whether there is a critical exposure window for the relationship between AT levels during pregnancy and the development of childhood asthma. Two studies conducted in California showed that AT exposure levels in the third trimester were associated with an increased risk of LBW (Basu et al. 2016) and fetal stillbirth (Basu et al. 2018). The results of both studies reported a sensitive exposure window for the association between AT and adverse pregnancy outcomes. Our further analysis revealed that the first trimester and the third trimester were observed as crucial windows in which prenatal extreme AT exposure affected asthma and wheezing in children aged 18 months to 3 years.
Interestingly, despite the fact that AT exposure levels in the first trimester were not colder in the case group than in the control group, the children developed asthma and wheezing. This may indicate that asthma and wheezing are the allergic constitution and are more susceptible to low temperatures. Although the mothers in the case group were not overexposed to low temperatures during pregnancy, their offspring showed hypersensitivity to extreme AT during embryonic life.
In addition, we discovered that exposure to extreme AT during pregnancy had a stronger impact on the development of childhood asthma and wheezing among children whose distance of residence was close to the nearest main traffic road, especially in the third trimester. Some studies have shown that people living in urban areas are more vulnerable, and living in wealthier urban areas may increase the risk of developing asthma, especially in children (DeVries et al. 2017). In our study, children whose mothers never took additional vitamin D supplements during pregnancy had a 28% increased risk of developing asthma and wheezing due to heat exposure (> 27.729 °C) in the third trimester compared to children whose mothers took vitamin D supplementation during pregnancy. Recent research revealed that maternal vitamin D deficiency can result in a variety of issues, including lower birth weight (Harvey et al. 2014), shorter gestation periods (Morley et al. 2006), and lower fetal blood calcium concentrations (Palermo and Holick 2014). Therefore, maternal vitamin D supplementation during pregnancy may be a smart preventive measure to avoid adverse fetal outcomes (Abreo et al. 2018; Kumar et al. 2022). We also observed that girls were more susceptible to the effects of extreme AT during the third trimester. Several studies have also reported that women are vulnerable to ambient temperature variations due to physiological factors (Yang et al. 2013; Zhou et al. 2014). Parental allergy was also found to be an effect modifier of extreme AT exposure on childhood asthma and wheezing. In the third trimester, compared to the no parental allergy group, the parental allergy group had a 1.5 times increased risk of childhood asthma and wheezing due to cold exposure (< 0.66 °C), and 2.7 times increased risk due to heat exposure (> 27.73 °C). Furthermore, we found an interaction between parental atopy and heat exposure during the third trimester on the risk of asthma development. Evidence suggested that both genetic and environmental factors contribute to asthma (Subbarao et al. 2009). A retrospective cohort study conducted in Ontario, Canada, found that a combination of maternal asthma and high levels of NO2 exposure during pregnancy can lead to the development of asthma in children (Lavigne et al. 2018). Parental allergy has been reported to be a risk factor for childhood asthma and may increase maternal sensitivity to environmental factors and exacerbate the effects of exposure to harmful environmental factors during pregnancy (Hansen et al. 2010). We hypothesize that this effect is due to increased inflammation during pregnancy, and our data supported the theory that the development of asthma in children is caused by a coordinated effect of genetic and environmental factors. More research like this is needed to fully explore the relationship and influence of genes and environment on disease.
Several mechanisms have been hypothesized to support the relationship between AT and childhood asthma and wheezing. For the possible mechanisms for heat-related effects on childhood asthma and wheezing, on the hand, acute heat stress might cause an inflammatory response (Wu et al. 2018) and influence the endocrine system (Regnault et al. 2007), which can result in placental anomalies. On the other hand, dehydration caused by maternal heat stress can lead to a decrease in fluid flow to the uterus, which can affect the blood supply to the fetus and cause asthma in children (Wang et al. 2020). For possible mechanisms associated with the effects of cold exposure on asthma and wheezing in children, some studies suggested that low temperatures can cause vasoconstriction, which restricts blood flow to the placenta and the fetus (Lian et al. 2017). Also, hypothermia has been related to peripheral vasoconstriction and hypertensive disorders in pregnancy in several studies, which could affect uteroplacental perfusion and harm the developing fetus (Bruckner et al. 2014). Some evidence supports our conclusions for the critical exposure period. It has been claimed that lung development starts at about the 4th gestational week, after which the airway begins to branch (Herriges and Morrisey 2014). During the third trimester of pregnancy, numerous terminal vesicles keep growing into primitive alveoli (Schittny 2017), and the development and maturation of the respiratory system would be harmed if exposed to extreme temperatures during this period. The exact biological mechanisms explaining the association between extreme temperatures during pregnancy and childhood asthma are unknown, and further research is needed.
It is worth mentioning that the prevalence of asthma and wheezing was 2.07% among children aged 18 months to 3 years, in our cross-sectional study. In a cohort study in the UK, the prevalence of preschool childhood wheezing was 7.7% (Bloom et al. 2021), and in a cross-sectional investigation in Shanghai, China, the overall incidence of asthma in children aged 3–7 years was 14.6% (Hu et al. 2021). Compared to other studies, our study had a lower prevalence of asthma and wheezing, which could be attributed to differences in the age group, economic status, and diagnostic criteria. In addition, since this study was conducted from December 2018 to March 2019, both maternal exposure during pregnancy and asthma observation were not affected by the COVID-19 epidemic. Considering that people who are advised to spend less time outdoors and wear masks whenever possible reduced the actual level of maternal exposure and hence reduced childhood asthma prevalence, the prevalence of asthma and wheezing reported in this study can be used as a prevalence before the COVID-19 epidemic, which can provide a reference for post-epidemic surveys about the prevalence of asthma and wheezing.
It is important to note that the grouped case-control study, one of the most commonly used case-control study methods (Shapiro 1982), was used in our study. The benefits of this approach in exploring the etiology of the disease are obvious. Firstly, the controls were randomly selected from the vaccination system before the start of the survey. The purpose of using this method is to avoid selection bias that might result from the use of convenience samples in vaccination clinics. Secondly, the cases and the controls were investigated simultaneously in our study, which greatly reduced possible information bias due to differences in investigators and the time. This is because the controls were sampled previously, and they could be investigated at the same time as screening the cases. Thirdly, the results of the pre-survey showed the prevalence of asthma in children was low. Based on this design, a large number of non-asthmatic children in our source population were not randomly sampled and did not all participate in the survey, which resulted in substantial cost savings. Finally, one of the objectives of this study was to identify possible modifiers in the relationship between AT and asthma and wheezing. For this purpose, the grouped case-control study is more suitable for observing the effect modifications of modifiers. However, some characteristics between the case and control groups were different because they were not individually matched, which was controlled in the multi-factor model, and a 1:1 match in the sensitivity analysis was performed to validate the stability of the results. However, there are some shortcomings in the grouped case-control study. For example, it reduced the efficiency of the study compared to the matched case-control study because far more controls were sampled than the number of cases.
This research has some advantages. First, we selected the AT as the research index, which combines ambient temperature, wind speed, and relative humidity and may be more objective than ambient temperature alone. Second, we used GAM and logistic regression model to analyze the effect of different AT exposure levels during pregnancy on asthma and wheezing in children, and we think that the two models may better show the association. Combining the rough effects observed by GAM and many previous studies (Gasparrini and Leone 2014), we divided the AT exposure levels during pregnancy into 3 levels; cold, moderate, and heat, which may deeply describe the relationship between AT during pregnancy and childhood asthma and wheezing. Third, the research was based on a cross-sectional study, and the cases and controls were recruited from the same source population, ensuring good comparability. Fourth, we separated the pregnancy into 4 time windows based on gestational weeks and then explored the independent effects of each time window on the outcomes in more detail. This research also has several disadvantages. First, there was a possibility of recall bias in the diagnosis of childhood asthma and other variables, because mother-children-pair data were gathered through maternal reports, although we controlled for children aged up to 3 years to reduce maternal recall bias. Second, even though several significant confounders were controlled as soon as possible, we cannot rule out the potential confounding factors, such as maternal obesity during pregnancy, which may cause errors in the association. Third, although this study investigated the effect of AT on childhood asthma and wheezing, we only considered outside temperature and ignored the effect of indoor temperature. This is something that has to be addressed in the follow-up research and more studies are needed to investigate the association between temperature and asthma in children.
Conclusions
The study indicates that exposure to extreme AT during the first trimester and the third trimester could increase the risk of childhood asthma and wheezing. Furthermore, we found this association may be affected by effect modification factors, such as children’s gender, the distance of residence from the nearest main traffic road, and parental atopy. These findings highlight the significance of conducting more research to validate the connections found here, and give further epidemiological research on the impact of meteorological conditions on childhood asthma during pregnancy.
Data availability
Due to privacy concerns of the cohort and subjects in the follow-up phase, the datasets created and/or analyzed during this study are not publically available. However, upon reasonable request, these can be obtained from the associated authors.
References
Abhijith KV, Kumar P, Gallagher J, McNabola A, Baldauf R, Pilla F, Broderick B, Di Sabatino S, Pulvirenti B (2017) Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments - a review. Atmos Environ 162:71–86. https://doi.org/10.1016/j.atmosenv.2017.05.014
Abreo A, Gebretsadik T, Stone CA, Hartert TV (2018) The impact of modifiable risk factor reduction on childhood asthma development. Clin Transl Med 7. https://doi.org/10.1186/s40169-018-0195-4
Akinbami LJ, Simon AE, Rossen LM (2016) Changing trends in asthma prevalence among children. Pediatrics 137. https://doi.org/10.1542/peds.2015-2354
Asher MI, Montefort S, Bjorksten B, Lai CKW, Strachan DP, Weiland SK, Williams H, Grp IPTS (2006) Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368:733–743. https://doi.org/10.1016/s0140-6736(06)69283-0
Asher MI, Stewart AW, Clayton T, Crane J, Ellwood PI, Mackay R, Mitchell E, Moyes C, Pattemore PK, Pearce N (2008) Has the prevalence and severity of symptoms of asthma changed among children in New Zealand? ISAAC Phase Three. N Z Med J 121:52–63
Avalos LA, Chen H, Li D-K, Basu R (2017) The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health 16. https://doi.org/10.1186/s12940-017-0209-5
Bai S, Zhao X, Liu Y, Lin S, Liu Y, Wang Z, Du S, Liu X, Wang Z (2022) The effect window for sulfur dioxide exposure in pregnancy on childhood asthma and wheezing: a case-control study. Environ Res 204. https://doi.org/10.1016/j.envres.2021.112286
Basagana X, Michael Y, Lensky IM, Rubin L, Grotto I, Vadislavsky E, Levi Y, Amitai E, Agay-Shay K (2021) Low and high ambient temperatures during pregnancy and birth weight among 624,940 singleton term births in Israel (2010–2014): an investigation of potential windows of susceptibility. Environ Health Perspect 129. https://doi.org/10.1289/ehp8117
Basu R, Sarovar V, Malig BJ (2016) Association between high ambient temperature and risk of stillbirth in California. Am J Epidemiol 183:894–901. https://doi.org/10.1093/aje/kwv295
Basu R, Rau R, Pearson D, Malig B (2018) Temperature and term low birth weight in California. Am J Epidemiol 187:2306–2314. https://doi.org/10.1093/aje/kwy116
Bloom CI, Franklin C, Bush A, Saglani S, Quint JK (2021) Burden of preschool wheeze and progression to asthma in the UK: population-based cohort 2007 to 2017. J Allergy Clin Immunol 147:1949–1958. https://doi.org/10.1016/j.jaci.2020.12.643
Bruckner TA, Modin B, Vagero D (2014) Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol 24:116–121. https://doi.org/10.1016/j.annepidem.2013.11.005
Buckley JP, Richardson DB (2012) Seasonal modification of the association between temperature and adult emergency department visits for asthma: a case-crossover study. Environ Health 11:6. https://doi.org/10.1186/1476-069x-11-55
Buteau S, Shekarrizfard M, Hatzopolou M, Gamache P, Liu L, Smargiassi A (2020) Air pollution from industries and asthma onset in childhood: a population-based birth cohort study using dispersion modeling. Environ Res 185:7. https://doi.org/10.1016/j.envres.2020.109180
Cong XW, Xu XJ, Zhang YL, Wang QH, Xu L, Huo X (2017) Temperature drop and the risk of asthma: a systematic review and meta-analysis. Environ Sci Pollut Res 24:22535–22546. https://doi.org/10.1007/s11356-017-9914-4
D’Amato G, Bergmann KC, Cecchi L, Annesi-Maesano I, Sanduzzi A, Liccardi G, Vitale C, Stanziola A, D’Amato M (2014) Climate change and air pollution: effects on pollen allergy and other allergic respiratory diseases. Allergo J Int 23:17–23
Davis CE (2013) Globalization, climate change, and human health. N Engl J Med 369:94–95. https://doi.org/10.1056/NEJMc1305749
DeVries A, Wlasiuk G, Miller SJ, Bosco A, Stern DA, Carla Lohman I, Rothers J, Jones AC, Nicodemus-Johnson J, Vasquez MM (2017) Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J Allergy Clin Immunol 140:534–542. https://doi.org/10.1016/j.jaci.2016.10.041
Gasparrini A, Leone M (2014) Attributable risk from distributed lag models. BMC Med Res Methodol 14. https://doi.org/10.1186/1471-2288-14-55
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73. https://doi.org/10.1056/NEJMra0708473
Hansen K, Mangrio E, Lindstrom M, Rosvall M (2010) Early exposure to secondhand tobacco smoke and the development of allergic diseases in 4 year old children in Malmo, Sweden. BMC Pediatr 10. https://doi.org/10.1186/1471-2431-10-61
Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E (2014) Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess 18:1. https://doi.org/10.3310/hta18450
Harville EW, Rabito FA (2018) Housing conditions and birth outcomes: the National Child Development Study. Environ Res 161:153–157. https://doi.org/10.1016/j.envres.2017.11.012
Hashimoto M, Fukuda T, Shimizu T, Watanabe S, Watanuki S, Eto Y, Urashima M (2004) Influence of climate factors on emergency visits for childhood asthma attack. Pediatr Int 46:48–52. https://doi.org/10.1111/j.1442-200X.2004.01835.x
Hernandez D (2016) Understanding ‘energy insecurity’ and why it matters to health. Soc Sci Med 167:1–10. https://doi.org/10.1016/j.socscimed.2016.08.029
Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141:502–513. https://doi.org/10.1242/dev.098186
Hsieh FY, Bloch DA, Larsen MD (1998) A simple method of sample size calculation for linear and logistic regression. Stat Med 17:1623–1634. https://doi.org/10.1002/(sici)1097-0258(19980730)17:14%3c1623::aid-sim871%3e3.0.co;2-s
Hsu HHL, Chiu YHM, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ (2015) Prenatal particulate air pollution and asthma onset in urban children identifying sensitive windows and sex differences. Am J Respir Crit Care Med 192:1052–1059. https://doi.org/10.1164/rccm.201504-0658OC
Hu Y-B, Chen Y-T, Liu S-J, Jiang F, Wu M-Q, Yan C-H, Tan J-G, Yu G-J, Hu Y, Yin Y (2021) Increasing prevalence and influencing factors of childhood asthma: a cross-sectional study in Shanghai, China. World J Pediatr 17:419–428. https://doi.org/10.1007/s12519-021-00436-x
Hughes MM, Black RE, Katz J (2017) 2500-g low birth weight cutoff: history and implications for future research and policy. Matern Child Health J 21:283–289. https://doi.org/10.1007/s10995-016-2131-9
Kaminsky DA, Bates JHT, Irvin CG (2000) Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am J Respir Crit Care Med 162:179–186. https://doi.org/10.1164/ajrccm.162.1.9806079
Kumar J, Kumar P, Goyal JP, Thakur C, Choudhary P, Meena J, Charan J, Singh K, Gupta A (2022) Vitamin D supplementation in childhood asthma: a systematic review and meta-analysis of randomised controlled trials. Erj Open Res 8. https://doi.org/10.1183/23120541.00662-2021
Lavigne E, Belair MA, Duque DR, Do MT, Stieb DM, Hystad P, van Donkelaar A, Martin RV, Crouse DL, Crighton E (2018) Effect modification of perinatal exposure to air pollution and childhood asthma incidence. Eur Resp J 51:13. https://doi.org/10.1183/13993003.01884-2017
Lee DS, Fahey DW, Skowron A, Allen MR, Burkhardt U, Chen Q, Doherty SJ, Freeman S, Forster PM, Fuglestvedt J (2021) The contribution of global aviation to anthropogenic climate forcing for 2000 to 2018. Atmos Environ 244. https://doi.org/10.1016/j.atmosenv.2020.117834
Lian S, Guo J, Wang L, Li W, Wang J, Ji H, Kong F, Xu B, Li S, Yang H (2017) Impact of prenatal cold stress on placental physiology, inflammatory response, and apoptosis in rats. Oncotarget 8:115304–115314. https://doi.org/10.18632/oncotarget.23257
Liu W, Huang C, Cai J, Wang X, Zou Z, Sun C (2018) Household environmental exposures during gestation and birth outcomes: a cross-sectional study in Shanghai, China. Sci Total Environ 615:1110–1118. https://doi.org/10.1016/j.scitotenv.2017.10.015
Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo YM, Tong SL, Coelho M, Saldiva PHN, Lavigne E, Matus P (2019) Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381:705–715. https://doi.org/10.1056/NEJMoa1817364
Lu C, Miao YF, Zeng J, Jiang W, Shen YM, Deng QH (2018) Prenatal exposure to ambient temperature variation increases the risk of common cold in children. Ecotoxicol Environ Saf 154:221–227. https://doi.org/10.1016/j.ecoenv.2018.02.044
Lu C, Zhang W, Zheng X, Sun J, Chen L, Deng Q (2020) Combined effects of ambient air pollution and home environmental factors on low birth weight. Chemosphere 240. https://doi.org/10.1016/j.chemosphere.2019.124836
Lu C, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Qian H, Wang J, Liu W, Sun Y (2022) Interaction effect of prenatal and postnatal exposure to ambient air pollution and temperature on childhood asthma. Environ Int 167. https://doi.org/10.1016/j.envint.2022.107456
McKie R (2013) Children will suffer most as climate change increases in coming decades, say scientists. BMJ-British Med J 347:1. https://doi.org/10.1136/bmj.f5799
Miao Y, Shen YM, Lu C, Zeng J, Deng Q (2017) Maternal exposure to ambient air temperature during pregnancy and early childhood pneumonia. J Therm Biol 69:288–293. https://doi.org/10.1016/j.jtherbio.2017.09.001
Miyake Y, Tanaka K, Fujiwara H, Mitani Y, Ikemi H, Sasaki S, Ohya Y, Hirota Y (2010) Residential proximity to main roads during pregnancy and the risk of allergic disorders in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 21:22–28. https://doi.org/10.1111/j.1399-3038.2009.00951.x
Morley R, Carlin JB, Pasco JA, Wark JD (2006) Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 91:906–912. https://doi.org/10.1210/jc.2005-1479
National Cooperative Group on Childhood A (2013) Institute of Environmental H, Related Product Safety C, Center for Disease C, Prevention, Chinese Center for Disease C, Prevention (2013) Third nationwide survey of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi = Chinese Journal of Pediatrics 51:729–735
O’Connor C, Livingstone V, Hourihane JOB, Irvine AD, Boylan G, Murray D (2022) Parental atopy and risk of atopic dermatitis in the first two years of life in the BASELINE birth cohort study. Pediatr Dermatol 39:896–902. https://doi.org/10.1111/pde.15090
Palermo NE, Holick MF (2014) Vitamin D, bone health, and other health benefits in pediatric patients. J Pediatr Rehabil Med 7:179–192. https://doi.org/10.3233/prm-140287
Regnault TRH, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G (2007) Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 28:714–723. https://doi.org/10.1016/j.placenta.2006.06.007
Rivera-González LO, Zhang Z, Sánchez BN, Zhang K, Brown DG, Rojas-Bracho L, Osornio-Vargas A, Vadillo-Ortega F (1995) O’Neill MS (2015) An assessment of air pollutant exposure methods in Mexico City, Mexico. J Air Waste Manag Assoc 65:581–591. https://doi.org/10.1080/10962247.2015.1020974
Schittny JC (2017) Development of the lung. Cell Tissue Res 367:427–444. https://doi.org/10.1007/s00441-016-2545-0
Shapiro S (1982) Case-control studies: design, conduct, analysis. JAMA 248(16):2055
Shirinde J, Wichmann J (2022) Temperature modifies the association between air pollution and respiratory disease mortality in Cape Town, South Africa. Int J Environ Health Res:1–10. https://doi.org/10.1080/09603123.2022.2076813
Steadman RG (1994) Norms of apparent temperature in Australia. Aust Meteorol Mag 43:1–16
Stevens CA, Turner D, Kuehni CE, Couriel JM, Silverman M (2003) The economic impact of preschool asthma and wheeze. Eur Resp J 21:1000–1006. https://doi.org/10.1183/09031936.03.00057002
Subbarao P, Becker A, Brook JR, Daley D, Mandhane PJ, Miller GE, Turvey SE, Sears MR, Investigators CS (2009) Epidemiology of asthma: risk factors for development. Expert Rev Clin Immunol 5:77–95. https://doi.org/10.1586/1744666x.5.1.77
Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P (2018) The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 52:3–12. https://doi.org/10.1016/j.bpobgyn.2018.04.003
Wang W (2016) Progress in the impact of polluted meteorological conditions on the incidence of asthma. J Thorac Dis 8:E57–E61. https://doi.org/10.3978/j.issn.2072-1439.2015.12.64
Wang Y-Y, Li Q, Guo Y, Zhou H, Wang Q-M, Shen H-P, Zhang Y-P, Yan D-H, Li S, Chen G (2020) Ambient temperature and the risk of preterm birth: a national birth cohort study in the mainland China. Environ Int 142. https://doi.org/10.1016/j.envint.2020.105851
Wu D, Lei J, Jia B, Xie H, Zhu Y, Xu J, Mori S, Zhang J, Burd I (2018) In vivo assessment of the placental anatomy and perfusion in a mouse model of intrauterine inflammation. J Magn Reson Imaging 47:1260–1267. https://doi.org/10.1002/jmri.25867
Xie M, Wenzel SE (2013) A global perspective in asthma: from phenotype to endotype. Chin Med J 126:166–174. https://doi.org/10.3760/cma.j.issn.0366-6999.20123023
Xu ZW, Huang CR, Hu WB, Turner LR, Su H, Tong SL (2013a) Extreme temperatures and emergency department admissions for childhood asthma in Brisbane, Australia. Occup Environ Med 70:730–735. https://doi.org/10.1136/oemed-2013-101538
Xu ZW, Huang CR, Su H, Turner LR, Qiao Z, Tong SL (2013b) Diurnal temperature range and childhood asthma: a time-series study. Environ Health 12:5. https://doi.org/10.1186/1476-069x-12-12
Yamazaki S, Shima M, Yoda Y, Oka K, Kurosaka F, Shimizu S, Takahashi H, Nakatani Y, Nishikawa J, Fujiwara K (2015) Exposure to air pollution and meteorological factors associated with children’s primary care visits at night due to asthma attack: case-crossover design for 3-year pooled patients. BMJ Open 5. https://doi.org/10.1136/bmjopen-2014-005736
Yang J, Liu H-Z, Ou C-Q, Lin G-Z, Zhou Q, Shen G-C, Chen P-Y, Guo Y (2013) Global climate change: impact of diurnal temperature range on mortality in Guangzhou, China. Environ Pollut 175:131–136. https://doi.org/10.1016/j.envpol.2012.12.021
Zheng XR, Kuang J, Lu C, Deng QH, Wu HY, Murithi RG, Johnson MB, Peng W, Wu ML (2021) Preconceptional and prenatal exposure to diurnal temperature variation increases the risk of childhood pneumonia. BMC Pediatr 21:8. https://doi.org/10.1186/s12887-021-02643-x
Zhou X, Zhao A, Meng X, Chen R, Kuang X, Duan X, Kan H (2014) Acute effects of diurnal temperature range on mortality in 8 Chinese cities. Sci Total Environ 493:92–97. https://doi.org/10.1016/j.scitotenv.2014.05.116
Acknowledgements
We would like to thank all of the children’s and parents’ cooperation, as well as all of the support and participation of clinics in our study. Also, we would like to thank the Jinan Center for Disease Control and Prevention’s coordination and assistance with the study, as well as the Jinan Ecological and Environmental Monitoring Center in Shandong Province’s providing of air pollution monitoring data.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81773386].
Author information
Authors and Affiliations
Contributions
Jiatao Zhang: conceptualization, formal analysis, writing—original draft. Shuoxin Bai: conceptualization, formal analysis, investigation, data curation, writing—review and editing. Shaoqian Lin: resources, investigation, data curation, writing—review and editing, validation. Liangliang Cui: resources, investigation. Xiaodong Zhao: investigation, resources. Shuang Du: conceptualization, methodology, data curation, validation. Zhiping Wang: project administration, supervision, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Preventive Medicine of Shandong University (approved number: 20170315).
Consent for publication
The author confirms that neither the entire manuscript nor any part of its content has been submitted for publication elsewhere. The author consents to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Bai, S., Lin, S. et al. Maternal apparent temperature during pregnancy on the risk of offspring asthma and wheezing: effect, critical window, and modifiers. Environ Sci Pollut Res 30, 62924–62937 (2023). https://doi.org/10.1007/s11356-023-26234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26234-8