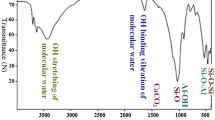
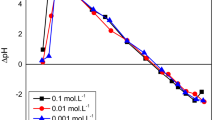
Abstract
Two superior adsorbents, namely bentonite and graphene oxide (GO), were hybridised to study the removal of copper and nickel ions from synthetic and industrial wastewater. The as-synthesised GO, bentonite/GO and bentonite were characterised by Fourier transform infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy and N2 adsorption-desorption analysis. The factors influencing the adsorption behaviours including contact time, initial solution pH, ionic strength, initial concentration of metal ions, temperature and adsorbent dosage were systematically investigated by batch equilibrium method. The adsorption equilibrium for copper and nickel onto bentonite was attained in 90 min while equilibrium was reached in 60 min on bentonite/GO. The adsorption of copper and nickel was pH-dependent in the range from pH 2 to pH 7 and from pH 2 to pH 8. Pseudo-first-order kinetic model excellently described the adsorption of copper and nickel onto bentonite and bentonite/GO. The equilibrium adsorption data was well described by the Langmuir isotherm model and the maximum adsorption capacity was 248.9 mg/g, 558.4 mg/g, 215.8 mg/g and 402.5 mg/g for bentonite-copper, bentonite/GO-copper, bentonite-nickel and bentonite/GO-nickel adsorption systems, respectively. The bentonite/GO composite exhibited a higher adsorption capacity of both cations from synthetic wastewater than pure bentonite owning to the synergistic effect between bentonite and GO. In all adsorption studies, copper was more efficiently removed than nickel due to its higher tendency to form bond with adsorbent surfaces. The adsorption of copper and nickel on bentonite/GO was mainly due to cation exchange, intermolecular and electrostatic interactions and physisorption dominated the adsorption processes. The practical application of bentonite/GO on adsorption of copper was investigated using real wastewater and its removal efficiency was beyond 98%. The excellent adsorption performances of composites for the copper and nickel removal from wastewater demonstrated its significant potential for pollution mitigations.


















Similar content being viewed by others
References
Ackley M, Rege S, Saxena H (2003) Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater 61:25–42
Adpakpang K, Oh SM, Park B, Hwang SJ (2016) Exfoliated clay nanosheets as an efficient additive for improving the electrode functionality of graphene-based nanocomposites. Inorg Chem Front 4(3):521–529
Akpomie KG, Dawodu FA (2015) Physicochemical analysis of automobile effluent before and after treatment with an alkaline-activated montmorillonite. Journal of Taibah University for Science 9.4:465–476
Al-Jariri J, Khalili F (2010) Adsorption of Zn(II), Pb(II), Cr(III) and Mn(II) from water by Jordanian bentonite. Desalin Water Treat 21:308–322
Al-Qunaibit MH, Mekhemer WK, Zaghloul AA (2005) The adsorption of Cu(II) ions on bentonite--a kinetic study. J Colloid Interface Sci 283:316–321
Al-Saydeh S, El-Naas M, Zaidi J (2017) Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem 56:35–44
Álvarez-Ayuso E, Sánchez A (2003) Removal of heavy metals from waste waters by natural and Na-exchanged bentonites. Clays Clay Min 51:475–480
Atun G, Sismanoglu T (1996) Adsorption of 4,4’ - isopropylidenediphenol and diphenylolpropane 4,4’ dioxyaceticacid from aqueous solution on kaolinite. J Environ Sci Health 31:2055–2069
Au PII, Leong Y-KK (2016) Surface chemistry and rheology of slurries of kaolinite and montmorillonite from different sources. Bulletin de l'Institut Scientifique, Section Sciences de la Terre (33): 17–32
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and Interpretation of Adsorption Isotherms. J Chem 3039817:11
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Belala Z, Jeguirim M, Belhachemi M, Addoun F, Trouvé G (2011) Biosorption of copper from aqueous solutions by date stones and palm-trees waste. Environ Chem Lett 9:65–69
Bourliva A, Michailidis K, Sikalidis C, Filippidis A, Betsiou M (2015) Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: study in mono-and multi-metal systems. Environ Earth Sci 73:5435–5444
Cao X, Yan B, Xue J, Wang Q, Wang Y, Huang Y, Zhang Y, Lyu X (2016) Effects of solution chemistry conditions and adsorbent surface properties on adsorption of Ni(II) on Laiyang bentonite. 18:66–75
Chai J, Au P, Mubarak NM et al (2020) Adsorption of heavy metal from industrial wastewater onto low-cost Malaysian kaolin clay–based adsorbent. Environ Sci Pollut Res 27:13949–13962
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001
Cheng H, Zeng K, Yu J (2013) Adsorption of uranium from aqueous solution by graphene oxide nanosheets supported on sepiolite. J Radioanal Nucl Chem 298:599–603
Chouchene A, Jeguirim M, Trouvé G (2014) Biosorption performance, combustion behavior, and leaching characteristics of olive solid waste during the removal of copper and nickel from aqueous solutions. Clean Techn Environ Policy 16:979–986
Ciesielczyk F, Bartczak P, Wieszczycka K, Siwińska-Stefańska K, Nowacka M, Jesionowski T (2013) Adsorption of Ni(II) from model solutions using co-precipitated inorganic oxides. Adsorption 19:423–434
Dawodu FA, Akpomie KG (2014) Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite clay. J Mater Res Technol 3:129–141
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Dhankhar R, Hooda A (2011) Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ Technol 32:467–491
Eluyemi M, Eleruja M, Adedeji A, Olofinjana B, Fasakin O, Akinwunmi O, Ilori O, Famojuro A, Ayinde S, Ajayi E (2016) Synthesis and characterization of graphene oxide and reduced graphene oxide thin films deposited by spray pyrolysis method. Graphene 5:143–154
Faria MCS, Rosemberg RS, Bomfeti CA, Monteiro DS, Barbosa F, Oliveira LCA, Rodriguez M, Pereira MC, Rodrigues JL (2014) Arsenic removal from contaminated water by ultrafine δ-FeOOH adsorbents. Chem Eng J 237:47–54
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Garcia H, Montes-Navajas P, Asenjo N, Santamaría R, Menendez R, Corma A (2013) Surface area measurement of graphene oxide in aqueous solutions. Langmuir 29(44):13443–13448
Ge Y, Li Z (2018) Application of lignin and its derivatives in adsorption of heavy metal ions in water: a review. ACS Sustain Chem Eng 6:7181–7192
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47:755–765
Green AA, Hersam MC (2010) Emerging methods for producing monodisperse graphene dispersions. J Phys Chem Lett 1:544–549
Guo H, White JC, Wang Z, Xing B (2018) Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr Opin Environ Sci Health 6:77–83
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. I&EC Fundam 5:212–223
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59(1):171–177
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Protect 76B:183–191
Hoor YQ, Au P-I, Mubarak N, Khalid M, Jagadish P, Walvekar R, Abdullah E (2020) Surface force arising from adsorbed graphene oxide in kaolinite suspensions. Colloids Surf A Physicochem Eng Asp 592:124592
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72
Jiang M-q, Jin X-y, Lu X-Q, Chen Z-l (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39
Karapinar N, Donat R (2009) Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination 249:123–129
Khan MA, Kim S-w, Rao RAK, Abou-Shanab RAI, Bhatnagar A, Song H, Jeon B-H (2010) Adsorption studies of dichloromethane on some commercially available GACs: effect of kinetics, thermodynamics and competitive ions. J Hazard Mater 178:963–972
Krstić V, Urošević T, Pešovski B (2018) A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem Eng Sci 192:273–287
Kubilay Ş, Gürkan R, Savran A, Şahan T (2007) Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 13:41–51
Langmuir I (1918) The adsorption of gasses on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Li J, Hu J, Sheng G, Zhao G, Huang Q (2009) Effect of pH, ionic strength, foreign ions and temperature on the adsorption of Cu(II) from aqueous solution to GMZ bentonite. Colloids Surf A Physicochem Eng Asp 349:195–201
Lian L, Guo L, Guo C (2009) Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J Hazard Mater 161:126–131
Liu Z-r, Zhou S-q (2010) Adsorption of copper and nickel on Na-bentonite. Process Saf Environ Prot 88:62–66
Liu H, Xie S, Liao J, Yan T, Liu Y, Tang X (2018) Novel graphene oxide/bentonite composite for uranium(VI) adsorption from aqueous solution. J Radioanal Nucl Chem 317:1349–1360
Malcolm RL, Kennedy VC (1969) Rate of cation exchange on clay minerals as determined by specific-ion electrode techniques1. Soil Sci Soc Am J 33:247–253
Marques J Jr, Lütke S, Frantz T, Espinelli J Jr, Carapelli R, Pinto L, Cadaval T Jr (2018) Removal of Al (III) and Fe (III) from binary system and industrial effluent using chitosan films. Int J Biol Macromol 120:1667–1673
Marques BS, Frantz TS, Junior TRSAC, de Almeida Pinto LA, Dotto GL (2019) Adsorption of a textile dye onto piaçava fibers: kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ Sci Pollut Res 26:28584–28592
Najafi F, Moradi O, Rajabi M, Asif M, Tyagi I, Agarwal S, Gupta VK (2015) Thermodynamics of the adsorption of nickel ions from aqueous phase using graphene oxide and glycine functionalized graphene oxide. J Mol Liq 208:106–113
Nasrullah A, Bhat AH, Isa MH (2016) Lignin: a sustainable biosorbent for heavy metal adsorption from wastewater, a review. AIP conference proceedings. Vol. 1787. No. 1. AIP Publishing LLC
Neelaveni M, Krishnan PS, Ramya R, Theres GS, Shanthi K (2019) Montmorillonite/graphene oxide nanocomposite as superior adsorbent for the adsorption of rhodamine B and nickel ion in binary system. Adv Powder Technol 30(3):596–609
Nyamunda B, Chivhanga T, Guyo U, Chigondo F (2019) Removal of Zn (II) and Cu (II) ions from industrial wastewaters using magnetic biochar derived from water hyacinth. J Eng 2019:1–11
Ojedokun AT, Bello OS (2016) Sequestering heavy metals from wastewater using cow dung. Water Resourc Indust 13:7–13
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504
Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjoeberg S, Wanner H (2007) Chemical speciation of environmentally significant metals with inorganic ligands - Part 2: The Cu2+-OH-, Cl-, CO32-, SO42-, and PO43- systems - (IUPAC technical report). Pure Appl Chem 79:950
Prasad M, Saxena S (2004) Sorption mechanism of some divalent metal ions onto low-cost mineral adsorbent. Ind Eng Chem Res 43:1512–1522
Prasad M, Xu H-y, Saxena S (2008) Multi-component sorption of Pb (II), Cu (II) and Zn (II) onto low-cost mineral adsorbent. J Hazard Mater 154:221–229
Rachou J, Gagnon C, Sauvé S (2007) Use of an ion-selective electrode for free copper measurements in low salinity and low ionic strength matrices. Environ Chem 4:90–97
Sen TK, Gomez D (2011) Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination 267:286–294
Sepulveda LA, Santana CC (2013) Effect of solution temperature, pH and ionic strength on dye adsorption onto Magellanic peat. Environ Technol 34:967–977
Sheng G, Yang S, Sheng J, Zhao D, Wang X (2011) Influence of solution chemistry on the removal of Ni(II) from aqueous solution to titanate nanotubes. Chem Eng J 168:178–182
Shirvani M, Rafiei HR, Bakhtiary S, Azimzadeh B, Amani S (2015) Equilibrium, kinetic, and thermodynamic studies on nickel removal from aqueous solutions using Ca-bentonite. Desalin Water Treat 54:464–472
Shukla A, Zhang Y-H, Dubey P, Margrave JL, Shukla SS (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95:137–152
Siddiqui SI, Chaudhry SA (2018) A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: application of isotherm, kinetic and thermodynamics. Process Saf Environ Prot 119:138–163
Ding S-l, Sun Y-z, Yang C-n, Xu B-h (2009) Removal of copper from aqueous solutions by bentonites and the factors affecting it. Min Sci Technol (China) 19:489–492
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47:9904–9910
Taha AA, Shreadah MA, Ahmed AM, Heiba HF (2016) Multi-component Adsorption of Pb(II), Cd(II), and Ni(II) onto Egyptian Na-activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J Environ Chem Eng 4(1):1166–180
Thajeel AS (2013) Isotherm, kinetic and thermodynamic of adsorption of heavy metal ions onto local activated carbon. Aquat Sci Technol 1(2):53–77
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Uddin M (2016) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Vafakhah S, Bahrololoom ME, Bazarganlari R, Saeedikhani M (2014) Removal of copper ions from electroplating effluent solutions with native corn cob and corn stalk and chemically modified corn stalk. J Environ Chem Eng 2:356–361
Vieira MGA, Neto AFA, Gimenes ML, da Silva MGC (2010) Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J Hazard Mater 177:362–371
Wanassi B, Hariz IB, Ghimbeu CM, Vaulot C, Hassen MB, Jeguirim M (2017) Carbonaceous adsorbents derived from textile cotton waste for the removal of Alizarin S dye from aqueous effluent: kinetic and equilibrium studies. Environ Sci Pollut Res 24:10041–10055
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
White RL, White CM, Turgut H, Massoud A, Tian ZR (2018) Comparative studies on copper adsorption by graphene oxide and functionalized graphene oxide nanoparticles. J Taiwan Inst Chem Eng 85:18–28
Wingenfelder U, Hansen C, Furrer G, Schulin R (2005) Removal of heavy metals from mine waters by natural zeolites. Environ Sci Technol 39:4606–4613
Xu W, Chen Y, Zhang W, Li B (2019) Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv Powder Technol 30:493–501
Yadav VB, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manag 232:803–817
Yang S, Li J, Lu Y, Chen Y, Wang X (2009) Sorption of Ni(II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl Radiat Isot 67:1600–1608
Yang S, Zhao D, Zhang H, Lu S, Chen L, Yu X (2010) Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J Hazard Mater 183:632–640
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, Gao B (2019) Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J 366:608–621
Zeng G, Liu Y, Tang L, Yang G, Pang Y, Zhang Y, Zhou Y, Li Z, Li M, Lai M, He X, He Y (2015) Enhancement of Cd(II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem Eng J 259:153–160
Zhang M (2011) Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem Eng J 172:361–368
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198-199:412–419
Zhou Y, Zhou L, Zhang X, Chen Y (2016) Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity. Microporous Mesoporous Mater 225:488–493
Zhu C, Dong X, Chen Z, Naidu R (2016) Adsorption of aqueous Pb(II), Cu(II), Zn(II) ions by amorphous tin(VI) hydrogen phosphate: an excellent inorganic adsorbent. Int J Environ Sci Technol 13(5):1257–1268
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, Y.S., Au, P.I., Mubarak, N.M. et al. Adsorption of Cu(II) and Ni(II) ions from wastewater onto bentonite and bentonite/GO composite. Environ Sci Pollut Res 27, 33270–33296 (2020). https://doi.org/10.1007/s11356-020-09423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09423-7