Abstract
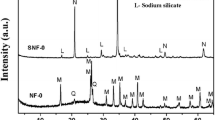
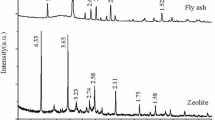
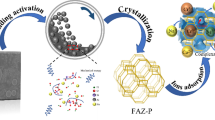
The present study aimed to synthesize Na-X zeolite from coal gangue powder (CGP) via the alkali fusion hydrothermal method. The optimal synthetic conditions were investigated, the mass ratio of CGP/NaOH(s) was 1:1.25, and crystallization reaction time was 12 h. X-ray powder diffraction, scanning electron microscopy energy-dispersive X-ray spectrum, and Fourier transform infrared spectrometer techniques were used to test the properties of resultant zeolite product, which was highly identical to that of commercial zeolite. The efficiencies of the synthetic zeolite for Pb2+ adsorption were analyzed on factors including solution pH, adsorbent dosage, temperature, and contact time. Compared with the pseudo-first-order, Elovich, Freundlich, and Temkin models, the pseudo-second-order and Langmuir models were fitted more satisfactorily with the dynamic data and adsorption equilibrium data, respectively. The maximum Pb2+ adsorption capacity of synthetic zeolite (457 mg/g) could be reached when the pH, contact time, temperature, and initial Pb2+ concentration was 6, 40 min, 45 °C, and 200 mg/L. The adsorption capacity was higher than many of the natural and synthetic zeolites reported in previous literature.









Similar content being viewed by others
Abbreviations
- CGP:
-
Coal gangue powder
- XRF:
-
X-ray fluorescence
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
- FTIR:
-
Fourier transform infrared
- FAAS:
-
Flame atomic adsorption spectrophotometer
- LOI:
-
Loss on ignition
References
Aldahri T, Behin J, Kazemian H, Rohani S (2016) Synthesis of zeolite Na-P from coal fly ash by thermo-sonochemical treatment. Fuel 182:494–501
Al-harahsheh MS, Zboom KA, Al-makhadmeh L, Hararah M, Mahasneh M (2015) Fly ash based geopolymer for heavy metal removal : a case study on copper removal. J Environ Chem Eng 3:1669–1677
Alijani H, Shariatinia Z (2017) Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 171:502–511
Alijani H, Hossein M, Shariatinia Z, Bayat M, Shemirani F (2014) A new approach for one step synthesis of magnetic carbon nanotubes / diatomite earth composite by chemical vapor deposition method : application for removal of lead ions. Chem Eng J 253:456–463
Alijani H, Shariatinia Z, Aroujalian A (2015) Water assisted synthesis of MWCNTs over natural magnetic rock : an effective magnetic adsorbent with enhanced mercury (II) adsorption property. Chem Eng J 281:468–481
Al-zboon K, Al-harahsheh MS, Bani F (2011) Fly ash-based geopolymer for Pb removal from aqueous solution. J Hazard Mater 188:414–421
Barbosa R, Lapa N, Lopes H, Günther A, Dias D, Mendes B (2014) Biomass fly ashes as low-cost chemical agents for Pb removal from synthetic and industrial wastewaters. J Colloid Interface Sci 424:27–36
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Bortone I, Di Nardo A, Di Natale M, Erto A, Musmarra D, Santonastaso GF (2013) Remediation of an aquifer polluted with dissolved tetrachloroethylene by an array of wells filled with activated carbon. J Hazard Mater 260:914–920
Donat R, Akdogan A, Erdem E, Cetisli H (2005) Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J Colloid Interface Sci 286:43–52
Doyle AM, Alismaeel ZT, Albayati TM, Abbas AS (2017) High purity FAU-type zeolite catalysts from shale rock for biodiesel production. Fuel 199:394–402
Faghihian H, Nourmoradi H, Shokouhi M (2012) Performance of silica aerogels modified with amino functional groups in Pb(II) and Cd(II) removal from aqueous solutions. Pol J Chem Technol 14:50–56
Gao Y, Huang H, Tang W, Liu X, Yang X, Zhang J (2015) Preparation and characterization of a novel porous silicate material from coal gangue. Microporous Mesoporous Mater 217:210–218
Gassowska M, Baranowska-Bosiacka I, Moczydłowska J, Tarnowski M, Pilutin A, Gutowska I, Struzynska L, Chlubek D, Adamczyk A (2016) Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3βand CDK5 dependent manner: relevance to neurological disorders. Toxicology 349:17–28
Haibin L, Zhenling L (2010) Resources, conservation and recycling recycling utilization patterns of coal mining waste in China. Resources, Conserv. Recycl 54:1331–1340
Ibrahim HS, Jamil TS, Hegazy EZ (2010) Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals : II. Isotherm models. J Hazard Mater 182:842–847
Khodadadi M, Malekpour A, Ansaritabar M (2017) Removal of Pb(II) and Cu (II) from aqueous solutions by NaA zeolite coated magnetic nanoparticles and optimization of method using experimental design. Microporous Mesoporous Mater 248:256–265
Kim SA, Kamala-Kannan S, Lee KJ, Park YJ, Shea PJ, Lee WH, Kim HM, Oh BT (2013) Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valent iron composite. Chem Eng J 217:54–60
Krishna BS, Murty DSR, Prakash BSJ (2000) Thermodynamics of chromium (VI) anionic species sorption onto surfactant-modified montmorillonite clay. J Colloid Interface Sci 229:230–236
Lee M, Park J, Kam S, Lee C (2018) Chemosphere synthesis of Na-A zeolite from Jeju Island scoria using fusion / hydrothermal method. Chemosphere 207:203–208
Li J, Wang J (2019) Comprehensive utilization and environmental risks of coal gangue: a review. J Clean Prod 239:117946
Li M, Wang S, Luo W, Xia H, Gao Q, Zhou C (2015) Facile synthesis and in situmagnetization of carbon-decorated lignocellulose fiber for highly efficient removal of methylene blue. J Chem Technol Biotechnol 90:1124–1134
Li X, Yan C, Luo W, Gao Q, Zhou Q, Liu C, Zhou S (2016) Exceptional cerium (III) adsorption performance of poly (acrylic acid) brushes-decorated attapulgite with abundant and highly accessible binding sites. Chem Eng J 284:333–342
Liu C, Liu Y, Ma Q, He H (2010) Mesoporous transition alumina with uniform pore structure synthesized by alumisol spray pyrolysis. Chem Eng J 163:133–142
Liu XD, Wang YP, Cui XM, He Y, Mao J (2013) Influence of synthesis parameters on NaA zeolite crystals. Powder Technol 243:184–193
Liu Y, Yan C, Zhang Z, Wang H, Zhou S, Zhou W (2016) A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel 185:181–189
Liu Y, Yan C, Zhao J, Zhang Z, Wang H, Zhou S, Wu L (2018) Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J Clean Prod 202:11–22
Liu C, Xia J, Fan H, Li W, Zheng G, Ma G, Liang Y (2020) Ti leaching differences during acid leaching of coal gangue based on different thermal fields. Waste Manag 101:66–73
Miyake M, Kimura Y, Ohashi T, Matsuda M (2008) Preparation of activated carbon – zeolite composite materials from coal fly ash. Microporous Mesoporous Mater 112:170–177
Murayama N, Yamamoto H, Shibata J (2002) Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int J Miner Process 64:1–17
Nekhunguni PM, Tavengwa NT, Tutu H (2017) Investigation of as (V) removal from acid mine drainage by iron (hydr) oxide modified zeolite. J Environ Manag 197:550–558
Ojha K, Pradhan NC, Samanta AN (2004) Zeolite from fly ash : synthesis and characterization. Bull Mater Sci 27:555–564
Pandey PK, Sharma SK, Sambi SS (2015) Removal of lead (II) from waste water on zeolite-NaX. J Environ Chem Eng 3:2604–2610
Qian T, Li J (2015) Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv Powder Technol 26:98–104
Qin L, Gao X (2019) Properties of coal gangue-Portland cement mixture with carbonation. Fuel 245:1–12
Reinoso D, Adrover M, Pedernera M (2018) Green synthesis of nanocrystalline faujasite zeolite. Ultrason Sonochem 42:303–309
Ren H, Jiang J, Wu D, Gao Z (2016) Selective adsorption of Pb (II) and Cr (VI) by surfactant-modified and unmodified natural zeolites: a comparative study on kinetics, equilibrium, and mechanism. Water Air Soil Pollut 227:3–11
Reynolds JG, Coronado PR, Hrubesh LW (2001) Hydrophobic aerogels for oil-spill cleanup – synthesis and characterization. J Non-Cryst Solids 292:127–137
Saltali K, Sarı A, Aydın M (2007) Removal of ammonium ion from aqueous solution by natural Turkish (Yıldızeli) zeolite for environmental quality. J Hazard Mater 141:258–263
Shariatinia Z, Bagherpour A (2018) Synthesis of zeolite NaY and its nanocomposites with chitosan as adsorbents for lead (II) removal from aqueous solution. Powder Technol 338:744–763
Sivalingam S, Sen S (2018) Rapid ultrasound assisted hydrothermal synthesis of highly pure nanozeolite X from fly ash for efficient treatment of industrial effluent. Chemosphere 210:816–823
Wang S, Ariyanto E (2007) Competitive adsorption of malachite green and Pb ions on natural zeolite. J Colloid Interface Sci 314:25–31
Wang C, Li J, Wang L, Sun X (2008) Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J Hazard Mater 155:58–64
Wang X, Shao D, Hou G, Wang X, Alsaedi A, Ahmad B (2015) Uptake of Pb(II) and U (VI) ions from aqueous solutions by the ZSM-5 zeolite adsorption percent (%). J Mol Liq 207:338–342
Wang J, Qin Q, Hu S, Wu K (2016) A concrete material with waste coal gangue and fly ash used for farmland drainage in high groundwater level areas. J Clean Prod 112:631–638
Wei P, Zhu X, Wang Y, Chu W, Xie S, Yang Z, Liu X, Li X, Xu L (2019) Rapid synthesis of ferrierite zeolite through microwave assisted organic template free route. Microporous Mesoporous Mater 279:220–227
Xie W, Zhou F, Bi X, Chen D, Li J, Sun S, Liu J, Chen X (2018) Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J Hazard Mater 358:441–449
Yang T, Han C, Liu H, Yang L, Liu D, Tang J, Luo Y (2019a) Synthesis of Na-X zeolite from low aluminum coal fly ash: characterization and high efficient As (V) removal. Adv Powder Technol 30:199–206
Yang L, Qian X, Yuan P, Bai H, Miki T, Men F, Li H, Nagasaka T (2019b) Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J Clean Prod 212:250–260
Zanin E, Scapinello J, De Oliveira M, Maria J, De Mello M, Antonio M, Oliveira JV, Dal J (2016) Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf Environ Prot 105:194–200
Zhang X, Tang D, Zhang M, Yang R (2013) Synthesis of NaX zeolite: influence of crystallization time, temperature and batch molar ratio SiO2/Al2O3 on the particulate properties of zeolite crystals. Powder Technol 235:322–328
Zhang H, Gu L, Zhang L (2017) Removal of aqueous Pb(II) by adsorption on Al2O3-pillared layered MnO2. Appl Surf Sci 406:330–338
Zhao Y, Zhang B, Zhang X, Wang J, Liu J, Chen R (2010) Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J Hazard Mater 178:658–664
Zhou C, Liu G, Wu S, Kwan P, Lam S (2014) The environmental characteristics of usage of coal gangue in bricking-making : a case study at Huainan, China. Chemosphere 95:274–280
Zhou J, Zheng F, Li H, Wang J, Bu N, Hu P, Gao J (2020) Optimization of post-treatment variables to produce hierarchical porous zeolites from coal gangue to enhance adsorption performance. Chem Eng J 381:122698
Funding
This work was financially supported by the Basic Research Program of Shanxi Province, China (No.201801D121267).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, Q., Moeen, M., Tian, Q. et al. Highly effective removal of Pb2+ in aqueous solution by Na-X zeolite derived from coal gangue. Environ Sci Pollut Res 27, 7398–7408 (2020). https://doi.org/10.1007/s11356-019-07412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07412-z