Abstract
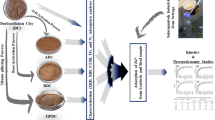
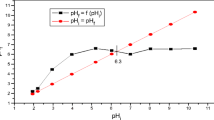
The fine fraction of the Tagaran natural clay (TC) from the Kurdistan region of Iraq-Sulaimani was characterized and used to remove Cd ions from industrial swage. Using XRF, XRD, SEM, and FTIR, the dominant clay mineral of the Tagaran clay mineral was identified as saponite, with minor amounts of chlorite. The clay was examined for its efficiency to adsorb and remove (Cd2+) in the presence of other heavy metal contaminants from Sulaimani industrial zone sewage by a batch method. The effect of initial pH, equilibrium time, temperature, clay dosage, and Cd2+ concentration was studied. Results were evaluated using Langmuir, Freundlich, Temkin, and Redlich-Peterson isotherms. The kinetics could be best fitted to pseudo-second-order reaction kinetic model. In addition, the activation energy and the amount of calculated and experimentally determined heavy metal loads were consistent. The thermodynamic studies showed spontaneous endothermic adsorption. The trioctahedral smectite (saponite) showed a good efficiency for the adsorption of Cd2+ from the real sample (up to 100%) which at least partly can be explained by cation exchange. Tagaran clay is a candidate material for the production of an adsorber material for removing Cd2+ from aqueous solutions.













Similar content being viewed by others
References
Abate G, Masini JC (2005) Influence of pH, ionic strength and humic acid on adsorption of Cd(II) and Pb(II) onto vermiculite. Colloids Surf A Physicochem Eng Asp 262:33–39
Abu, H. and Moussab, H. (2004) Removal of heavy metals from wastewater by membrane processes : a comparative study. 164, 105–110
Ahmed, H.R., Raheem, S.J., and Aziz, B.K. (2017) Removal of Leishman stain from aqueous solutions using natural clay of Qulapalk area of Kurdistan region of Iraq. Karbala International Journal of Modern Science, 3, 165–175. Elsevier ltd.
Alkan M, Doǧan M, Turhan Y, Demirbaş Ö, Turan P (2008) Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions. Chem Eng J 139:213–223
Anna J, Hoek EMV (2010) Removing cadmium ions from water via nanoparticle-enhanced ultrafiltration. Environ Sci Technol 44:2570–2576
Appel J (1973) Freundlich's adsorption isotherm. Surf Sci 39:237–244
Arab M, Bougeard D, Smirnov KS (2002) Experimental and computer simulation study of the vibrational spectra of vermiculite. Phys Chem Chem Phys 4:1957–1963
Areco MM, Afonso S (2010) Colloids and surfacesB: biointerfaces copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus. Thermodynamics and Kinetics Studies 81:620–628
Asgari M, Anisi H, Mohammadi H, Sadighi S (2014) Designing a commercial scale pressure swing adsorber for hydrogen purification. Petroleum and Coal 56:552–561
Aziz BK, Abdullah MA, Jubrael KJ (2011) Acid activation and bleaching capacity of some clays for decolourizing used oils. Asian J Chem
Baek MH, Ijagbemi CO, O SJ, Kim DS (2010) Removal of malachite green from aqueous solution using degreased coffee bean. J Hazard Mater 176:820–828
Basci, N., Kocadagistan, E., and Kocadagistan, B. (2004) Biosorption of copper(II) from aqueous solutions by wheat shell. 164, 135–140
Bel, H., Sdiri, A., Ltaief, W., Da, P., Ben, M., and Galves, M.E. (2017) Comptes Rendus Chimie Efficient removal of cadmium and 2-chlorophenol in aqueous systems by natural clay: adsorption and photo-Fenton degradation processes
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf Sci 140:114–131
Çay S, Uyanik A, Özaşik A (2004) Single and binary component adsorption of copper(II) andcadmium(II) from aqueous solutions using tea-industry waste. Sep Purif Technol 38:273–280
Chamsaz M, Atarodi A, Eftekhari M, Asadpour S, Adibi M (2013) Vortex-assisted ionic liquid microextraction coupled to flame atomic absorption spectrometry for determination of trace levels of cadmium in real samples. J Adv Res 4:35–41 Cairo University
Chandra, G.P., Satyaveni, S., Ramesh, A., Seshaiah, K., Murthy, K.S.N., and Choudary, N. V. (2006) Sorption of cadmium and zinc from aqueous solutions by zeolite 4A , zeolite 13X and bentonite. 81, 265–272
Charerntanyarak, L. (1999) Heavy metals removal by chemical coagulation and. Water Science and Technology, 39, 135–138. International association on water quality
Dal Bosco SM, Jimenez RS, Vignado C, Fontana J, Geraldo B, Figueiredo FCA, Mandelli D, Carvalho WA (2006) Removal of Mn(II) and Cd(II) from wastewaters by natural and modified clays. Adsorption 12:133–146
Ding, F., Gao, M., Wang, J., Shen, T., and Zang, W. (2017) Tuning wettability by controlling the layer charge and structure of organo-vermiculites. Journal of Industrial and Engineering Chemistry. The Korean Society of Industrial and Engineering Chemistry
Dohrmann R, Genske D, Karnland O, Kaufhold S, Kiviranta L, Olsson S, Plötze M, Sandén T, Sellin P, Svensson D, Valter M (2012) Interlaboratory CEC and exchangeable cation study of bentonite buffer materials: I. Cu(II)-triethylenetetramine method. Clay Clay Miner 60:162–175
Eloussaief M, Sdiri A, Benzina M (2013) Modelling the adsorption of mercury onto natural and aluminium pillared clays. Environ Sci Pollut Res 20:469–479
Erdem, E., Karapinar, N., and Donat, R. (2004) The removal of heavy metal cations by natural zeolites. 280, 309–314
Frantz TS, Silveira N, Quadro MS, Andreazza R, Barcelos AA, Cadaval TRS, Pinto LAA (2017) Cu(II) adsorption from copper mine water by chitosan films and the matrix effects. Environ Sci Pollut Res 24:5908–5917
Gopal, K. and Sen, S. (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. 140, 114–131
Gupta SS, Bhattacharyya KG (2006) Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives. J Hazard Mater 128:247–257
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Sen Gupta S, Bhattacharyya KG (2008) Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manag 87:46–58
Hu C, Zhu P, Cai M, Hu H, Fu Q (2017) Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: kinetic, thermodynamic and equilibrium studies. Appl Clay Sci 143:320–326 Elsevier
Hwang, D.F. and Wang, L.C. (2001) Effect of taurine on toxicity of cadmium in rats. 167, 173–180
Ismadji, S., Soetaredjo, F.E., and Ayucitra, A. (2015) Clay materials for environmental remediation. P. in.:
Jiang MQ, Jin XY, Lu XQ, Chen ZL (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39 Elsevier BV
Johnson BB (1990) Effect of pH, temperature, and concentration on the adsorption of cadmium on goethite. Environ Sci Technol 24:112–118
Kumar U, Bandyopadhyay M (2006) Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour Technol 97:104–109
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lasheen, M.R., El-sherif, I.Y., and El-wakeel, S.T. (2017) Heavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocomposite. 8, 503–511
Lin, S. and Juang, R. (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. 92, 315–326
Malkoc E, Nuhoglu Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of Cr(VI) onto waste acorn of Quercus ithaburensis. Chem Eng Process Process Intensif 46:1020–1029
Marjanović V, Lazarević S, Janković-Častvan I, Jokić B, Janaćković D, Petrović R (2013) Adsorption of chromium(VI) from aqueous solutions onto amine-functionalized natural and acid-activated sepiolites. Appl Clay Sci 80–81:202–210
Meunier, N., Laroulandie, J., Blais, J.F., and Tyagi, R.D. (2003) Cocoa shells for heavy metal removal from acidic solutions. 90, 255–263
Mosser-Ruck R, Devineau K, Charpentier D, Cathelineau M (2005) Effects of ethylene glycol saturation protocols on XRD patterns: a critical review and discussion. Clay Clay Miner 53:631–638
Motokawa R, Endo H, Yokoyama S, Ogawa H, Kobayashi T, Suzuki S, Yaita T (2014) Mesoscopic structures of vermiculite and weathered biotite clays in suspension with and without cesium ions. Langmuir 30:15127–15134
Muiambo HF, Focke WW, Atanasova M, van der Westhuizen I, Tiedt LR (2010) Thermal properties of sodium-exchanged palabora vermiculite. Appl Clay Sci 50:51–57 Elsevier B.V
Ngah WSW, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456 Elsevier ltd.
Ondo JA (2010) Evaluation of the absorption capacity of the natural clay from Bikougou (Gabon) to remove Mn (II) from aqueous solution. Int J Eng Sci Technol 2:5001–5016
Ozdes D, Duran C, Senturk HB (2011) Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay. J Environ Manag 92:3082–3090 Elsevier ltd.
Pardo L, Cecilia J, López-Moreno C, Hernández V, Pozo M, Bentabol M, Franco F (2018) Influence of the structure and experimental surfaces modifications of 2:1 clay minerals on the adsorption properties of methylene blue. Minerals 8:359
Pazourková, L., Martynková, G.S., Hundáková, M., and Barošová, H. (2014) M Ontmorillonite and vermiculite modified by N-vinylcaprolactam and poly (N-vinylcaprolactam) preparation and characterization. 1–6
Purna Chandra Rao G, Satyaveni S, Ramesh A, Seshaiah K, Murthy KSN, Choudary NV (2006) Sorption of cadmium and zinc from aqueous solutions by zeolite 4A, zeolite 13X and bentonite. J Environ Manag 81:265–272
Rao RAK, Kashifuddin M (2016) Adsorption studies of Cd(II) on ball clay: comparison with other natural clays. Arab J Chem 9:S1233–S1241 King Saud University
Roushani, M., Saedi, Z., and Baghelani, Y.M. (2017) Environmental nanotechnology, monitoring & management removal of cadmium ions from aqueous solutions using TMU-16-NH 2 metal organic framework. Environmental Nanotechnology, Monitoring & Management, 7, 89–96. Environmental Nanotechnology, Monitoring & Management
Sun Q, Liu C, Cui P, Fan T, Zhu M, Alves ME, Siebecker MG, Sparks DL, Wu T, Li W, Zhou D, Wang Y (2019) Formation of Cd precipitates on Γ-Al 2 O 3: implications for Cd sequestration in the environment. Environ Int 126:234–241 Elsevier
Sutcu M (2015) Influence of expanded vermiculite on physical properties and thermal conductivity of clay bricks. Ceram Int 41:2819–2827 Elsevier
Tor A, Cengeloglu Y (2006) Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater 138:409–415
Veli, S. and Aly, B. (2007) Adsorption of copper and zinc from aqueous solutions by using natural clay. 149, 226–233
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135
Xu, L., Zheng, X., Cui, H., Zhu, Z., Liang, J., and Zhou, J. (2017) Equilibrium, kinetic, and thermodynamic studies on the adsorption of cadmium from aqueous solution by modified biomass ash. 2017. Hindawi Publishing Corporation
Acknowledgments
We gratefully acknowledge Federal Institute for Geosciences and Natural Resources (BGR), for the support and their assistance and all who contributed to conduction of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aziz, B.K., Shwan, D.M.S. & Kaufhold, S. Characterization of Tagaran natural clay and its efficiency for removal of cadmium (II) from Sulaymaniyah industrial zone sewage. Environ Sci Pollut Res 27, 38384–38396 (2020). https://doi.org/10.1007/s11356-019-06995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06995-x