Abstract
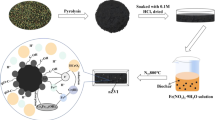
The chitosan-stabilized ferrous sulfide nanoparticles were loaded on biochar to prepare a composite material FeS-CS-BC for effective removal of hexavalent chromium in water. BC and FeS-CS-BC were characterized by Brunauer–Emmett–Teller (BET), scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR) analyses. Batch experiments were employed to evaluate the Cr(VI) removal performance. The experimental results showed that the removal rate of Cr(VI) by FeS-CS-BC(FeS:CS:BC = 2:2:1) reached 98.34%, which was significantly higher than that of BC (44.58%) and FeS (79.91%). In the pH range of 2–10, the removal of Cr(VI) by FeS-CS-BC was almost independent of pH. The limitation of coexisting anions (Cl−、SO42−、NO3−) on Cr(VI) removal was not too obvious. The removal of Cr(VI) by FeS-CS-BC was fitted with the pseudo-second-order dynamics, which was a hybrid chemical-adsorption reaction. The X-ray photoelectron spectroscopy (XPS) analysis result showed that Cr(VI) was reduced, and the reduced Cr(VI) was fixed on the surface of the material in the form of Cr(VI)–Fe(III).

Removal of hexavalent chromium from wastewater by FeS-CS-BC composite synthesized by impregnation.








Similar content being viewed by others
References
Ahn H, Jo HY, Kim G-Y, Koh Y-K (2012) Effect of NaCl on Cr(VI) reduction by granular zero valent iron (ZVI) in aqueous solutions. Mater Trans 53:1324–1329
An Q, Li XQ, Nan HY, Yu Y, Jiang JN (2018) The potential adsorption mechanism of the biochars with different modification processes to Cr(VI). Environ Sci Pollut Res Int 25:31346–31357
Calagui MJ, Senoro DB, Kan CC, Salvacion JW, Futalan CM, Wan MW (2014) Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J Hazard Mater 277:120–126
Cao W, Wang Z, Ao H, Yuan B (2018) Removal of Cr(VI) by corn stalk based anion exchanger: the extent and rate of Cr(VI) reduction as side reaction. Colloids Surf A Physicochem Eng Asp 539:424–432
Dong C, Chen W, Liu C (2014) Preparation of novel magnetic chitosan nanoparticle and its application for removal of humic acid from aqueous solution. Appl Surf Sci 292:1067–1076
Dong Z, Zhao J, Du J, Li C, Zhao L (2016) Radiation synthesis of spherical cellulose-based adsorbent for efficient adsorption and detoxification of Cr(VI). Radiat Phys Chem 126:68–74
Elangovan R, Philip L, Chandraraj K (2008) Biosorption of chromium species by aquatic weeds: kinetics and mechanism studies. J Hazard Mater 152:100–112
Fan Z, Zhang Q, Gao B, Li M, Liu C, Qiu Y (2019) Removal of hexavalent chromium by biochar supported nZVI composite: batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 217:85–94
Geng B, Jin Z, Li T, Qi X (2009) Preparation of chitosan-stabilized Fe(0) nanoparticles for removal of hexavalent chromium in water. Sci Total Environ 407:4994–5000
Gong Y, Liu Y, Xiong Z, Kaback D, Zhao D (2012) Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 23:294007
Gong Y, Liu Y, Xiong Z, Zhao D (2014) Immobilization of mercury by carboxymethyl cellulose stabilized iron sulfide nanoparticles: reaction mechanisms and effects of stabilizer and water chemistry. Environ Sci Technol 48:3986–3994
Gopal Reddi MR, Gomathi T, Saranya M, Sudha PN (2017) Adsorption and kinetic studies on the removal of chromium and copper onto chitosan-g-maliec anhydride-g-ethylene dimethacrylate. Int J Biol Macromol 104:1578–1585
Han YS, Gallegos TJ, Demond AH, Hayes KF (2011) FeS-coated sand for removal of arsenic(III) under anaerobic conditions in permeable reactive barriers. Water Res 45:593–604
Huang L, Xue J, Jin F, Zhou S, Wang M, Liu Q, Huang L (2014) Study on mechanism and influential factors of the adsorption properties and regeneration of activated carbon fiber felt (ACFF) for Cr(VI) under electrochemical environment. J Taiwan Inst Chem Eng 45:2986–2994
Hyun SP, Davis JA, Sun K, Hayes KF (2012) Uranium(VI) reduction by iron(II) monosulfide mackinawite. Environ Sci Technol 46:3369–3376
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman AR (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresour Technol 101:8868–8872
Kustov LM, Finashina ED, Shuvalova EV, Tkachenko OP, Kirichenko OA (2011) Pd-Fe nanoparticles stabilized by chitosan derivatives for perchloroethene dechlorination. Environ Int 37:1044–1052
Liu WJ, Tian K, Jiang H, Yu HQ (2013) Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci Rep 3:2419
Liu T, Gao B, Fang J, Wang B, Cao X (2016) Biochar-supported carbon nanotube and graphene oxide nanocomposites for Pb(ii) and Cd(ii) removal. RSC Adv 6:24314–24319
Lyu H, Tang J, Huang Y, Gai L, Zeng EY, Liber K, Gong Y (2017) Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem Eng J 322:516–524
Maity J, Ray SK (2018) Chitosan based nano composite adsorbent-synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr Polym 182:159–171
Mandal S, Sarkar B, Bolan N, Ok YS, Naidu R (2017) Enhancement of chromate reduction in soils by surface modified biochar. J Environ Manag 186:277–284
Mullet M, Boursiquot S, Ehrhardt J-J (2004) Removal of hexavalent chromium from solutions by mackinawite, tetragonal FeS. Colloids Surf A Physicochem Eng Asp 244:77–85
Peng Z, Zhao H, Lyu H, Wang L, Huang H, Nan Q, Tang J (2018) UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environ Sci Pollut Res Int 25:10808–10819
Qi W, Zhao Y, Zheng X, Ji M, Zhang Z (2016) Adsorption behavior and mechanism of Cr(VI) using Sakura waste from aqueous solution. Appl Surf Sci 360:470–476
Ren G, Wang X, Huang P, Zhong B, Zhang Z, Yang L, Yang X (2017) Chromium (VI) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci Total Environ 607-608:900–910
Rivero-Huguet M, Marshall WD (2009) Reduction of hexavalent chromium mediated by micro- and nano-sized mixed metallic particles. J Hazard Mater 169:1081–1087
Shen YS, Wang SL, Tzou YM, Yan YY, Kuan WH (2012) Removal of hexavalent Cr by coconut coir and derived chars—the effect of surface functionality. Bioresour Technol 104:165–172
Shi S, Yang J, Liang S, Li M, Gan Q, Xiao K, Hu J (2018) Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci Total Environ 628-629:499–508
Skyllberg U, Drott A (2010) Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)—an EXAFS study. Environ Sci Technol 44:1254–1259
Su C, Puls RW (2001) Arsenate and arsenite removal by zerovalent iron: effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ Sci Technol 35:4562–4568
Sun M, Cheng G, Ge X, Chen M, Wang C, Lou L, Xu X (2018) Aqueous Hg(II) immobilization by chitosan stabilized magnetic iron sulfide nanoparticles. Sci Total Environ 621:1074–1083
Troiano JM, Jordan DS, Hull CJ, Geiger FM (2013) Interaction of Cr(III) and Cr(VI) with hematite studied by second harmonic generation. J Phys Chem C 117:5164–5171
Vijayakumar R, Koltypin Y, Felner I, Gedanken A (2000) Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater Sci Eng A286:101–105
Wang H, Feng M, Zhou F, Huang X, Tsang DCW, Zhang W (2017a) Effects of atmospheric ageing under different temperatures on surface properties of sludge-derived biochar and metal/metalloid stabilization. Chemosphere 184:176–184
Wang S, Gao B, Li Y, Creamer AE, He F (2017b) Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: batch and continuous flow tests. J Hazard Mater 322:172–181
Wang T, Liu Y, Wang J, Wang X, Liu B, Wang Y (2019) In-situ remediation of hexavalent chromium contaminated groundwater and saturated soil using stabilized iron sulfide nanoparticles. J Environ Manag 231:679–686
Weng X, Lin S, Zhong Y, Chen Z (2013) Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chem Eng J 229:27–34
Wolthers M, Charlet L, van Der Weijden CH, van der Linde PR, Rickard D (2005) Arsenic mobility in the ambient sulfidic environment: sorption of arsenic(V) and arsenic(III) onto disordered mackinawite. Geochim Cosmochim Acta 69:3483–3492
Wu J, Zeng RJ (2018) In situ preparation of stabilized iron sulfide nanoparticle-impregnated alginate composite for selenite remediation. Environ Sci Technol 52:6487–6496
Wu J, Wang XB, Zeng RJ (2017) Reactivity enhancement of iron sulfide nanoparticles stabilized by sodium alginate: taking Cr (VI) removal as an example. J Hazard Mater 333:275–284
Xie Y, Yi Y, Qin Y, Wang L, Liu G, Wu Y, Diao Z, Zhou T, Xu M (2016) Perchlorate degradation in aqueous solution using chitosan-stabilized zero-valent iron nanoparticles. Sep Purif Technol 171:164–173
Xu C, Yang W, Liu W, Sun H, Jiao C, Lin AJ (2018) Performance and mechanism of Cr(VI) removal by zero-valent iron loaded onto expanded graphite. J Environ Sci 67:14–22
Yang R, Wang Y, Li M, Hong Y (2014) A new carbon/ferrous sulfide/iron composite prepared by an in situ carbonization reduction method from hemp (Cannabis sativa L.) stems and its Cr(VI) removal ability. ACS Sustain Chem Eng 2:1270–1279
Yen M-Y, Teng C-C, Hsiao M-C, Liu P-I, Chuang W-P, Ma C-CM, Hsieh C-K, Tsai M-C, Tsai C-H (2011) Platinum nanoparticles/graphene composite catalyst as a novel composite counter electrode for high performance dye-sensitized solar cells. J Mater Chem 21:12880
Yin X, Liu W, Ni J (2014) Removal of coexisting Cr(VI) and 4-chlorophenol through reduction and Fenton reaction in a single system. Chem Eng J 248:89–97
Yu J, Jiang C, Guan Q, Ning P, Gu J, Chen Q, Zhang J, Miao R (2018) Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 195:632–640
Yuan W, Xu W, Wu Z, Zhang Z, Wang L, Bai J, Wang X, Zhang Q, Zhu X, Zhang C, Wang J (2018) Mechanochemical treatment of Cr(VI) contaminated soil using a sodium sulfide coupled solidification/stabilization process. Chemosphere 212:540–547
Zhang Y, Li Y, Li J, Sheng G, Zhang Y, Zheng X (2012) Enhanced Cr(VI) removal by using the mixture of pillared bentonite and zero-valent iron. Chem Eng J 185-186:243–249
Zhang H, Peng L, Chen A, Shang C, Lei M, He K, Luo S, Shao J, Zeng Q (2019) Chitosan-stabilized FeS magnetic composites for chromium removal: characterization, performance, mechanism, and stability. Carbohydr Polym 214:276–285
Zhou Y, Gao B, Zimmerman AR, Fang J, Sun Y, Cao X (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518
Zhou Y, Gao B, Zimmerman AR, Chen H, Zhang M, Cao X (2014) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152:538–542
Zhu S, Huang X, Wang D, Wang L, Ma F (2018) Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: mechanisms and application potential. Chemosphere 207:50–59
Funding
This work was supported by the National Key Research and Development Program of China (2017YFD0801503) and the National Natural Science Foundation of China (41701367, 41877133).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• FeS-CS-BC was synthesized combining the advantages of FeS, chitosan, and biochar.
• BC, FeS, and FeS-CS-BC were employed to assess Cr(VI) removal performance.
• The modified material with FeS:CS:BC=2:2:1 is the best experimental material for the study.
• The application of FeS-CS-BC is greatly limited under alkaline conditions.
• Cr(VI) isotherm adsorption data conforms to Redlich-Peterson model.
Electronic supplementary material
ESM 1
(DOCX 170 kb)
Rights and permissions
About this article
Cite this article
Qin, L., He, L., Yang, W. et al. Preparation of a novel iron-based biochar composite for removal of hexavalent chromium in water. Environ Sci Pollut Res 27, 9214–9226 (2020). https://doi.org/10.1007/s11356-019-06954-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06954-6