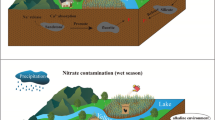
Abstract
Trifluoroacetic acid (TFA) is a ubiquitous and extremely stable contaminant in the ambient environment and may be discharged during fluorochemical production processes. However, the impacts of fluorochemical production on surrounding areas have seldom been evaluated. We focused on Jinan, the capital of Shandong Province, China, and measured TFA levels in water, soil, and air samples. Our results showed that the average TFA concentrations in flowing water bodies were lower than those in landscape water bodies. The average TFA concentrations in soils were significantly higher than the background concentration. As for atmospheric TFA levels, the mean concentrations in the gas phase were higher than those in the particle phase, and average daytime levels were slightly higher than nighttime levels. In addition, the quotient method was used to assess the ecological risk of TFA in water in Jinan. The ratio of pollutant environmental concentration to predicted no-effect concentration (PEC/PNEC) for TFA was greater than 1, indicating that TFA does potentially damage the aquatic ecosystem of Jinan. Our findings suggest that TFA pollution around fluoride production plants is a serious problem and that actions are required to avoid exacerbating the local ecological and environmental risks of TFA.



Similar content being viewed by others
References
Berends AG, Boutonnet JC, Rooij CGD, Thompson RS (1999) Toxicity of trifluoroacetate to aquatic organisms. Environ Toxicol Chem 18(5):1053–1059
Berg M, Müller SR, Mühlemann J, Wiedmer A, Schwarzenbach RP (2000) Concentrations and mass fluxes of chloroacetic acids and trifluoroacetic acid in rain and natural waters in Switzerland. Environ Sci Technol 34(13):2675–2683
Boutonnet JC, Bingham P, Calamari D, Rooij CD, Franklin J, Kawano T, Libre JM, McCulloch A, Malinverno G, Odom JM (1999) Environmental risk assessment of trifluoroacetic acid. Human Ecol Risk Assess: Int J 5(1):59–124
Davison A, Pearson S (1997) Toxicity of TFA to plants. Final report to AFEAS by the University of Newcastle (Project SP91–18.23/BP96–31).
Ellis DA, Hanson ML, Sibley PK, Shahid T, Fineberg NA, Solomon KR, Muir DC, Mabury SA (2001a) The fate and persistence of trifluoroacetic and chloroacetic acids in pond waters. Chemosphere 42(3):309–318
Ellis DA, Mabury SA, Martin JW, Muir DCG (2001b) Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 412(6844):321–324
EU (2003) Technical Guidance Document (TGD) on Risk Assessment of Chemical Substances following European Regulations and Directives, Parts I-IV. Technical Report Number EUR 20418 EN/1-4.
Frank H, Renschen D, Klein A, Scholl H (1995) Trace analysis of airborne haloacetates. J Sep Sci 18(2):83–88
Guo J, Zhai Z, Wang L, Wang Z, Wu J, Zhang B, Zhang J (2017) Dynamic and thermodynamic mechanisms of TFA adsorption by particulate matter. Environ Pollut 225:175–183
Han W, Kennedy EM, Kundu SK, Mackie JC, Adesina AA, Dlugogorski BZ (2010) Experimental and chemical kinetic study of the pyrolysis of trifluoroethane and the reaction of trifluoromethane with methane. J Fluor Chem 131(7):751–760
Han W, Jin B, Zhou Q, Wang S (2014) Conversion and resource utilization of waste CHF3 gas. Chem Ind Eng Prog 33(2):483–492 (in Chinese)
Hanson ML, Solomon KR (2004) Haloacetic acids in the aquatic environment. Part I: macrophyte toxicity. Environ Pollut 130(3):371–383
Kanaya Y, Cao R, Akimoto H, Fukuda M, Komazaki Y, Yokouchi Y, Koike M, Tanimoto H, Takegawa N, Kondo Y (2007) Urban photochemistry in central Tokyo: 1. Observed and modeled OH and HO2 radical concentrations during the winter and summer of 2004. J Geophys Res: Atmos 112(D21)
Kazil J, Mckeen SA, Kim S, Ahmadov R, Grell GA, Talukdar RK, Ravishankara AR (2011) WRF/Chem study of dry and wet deposition of trifluoroacetic acid produced from the atmospheric degradation of a few short-lived HFCs, AGU Fall Meeting
Key BD, Howell RD, Criddle CS (1997) Fluorinated organics in the biosphere. Environ Sci Technol 31(9):2445–2454
Lu K, Hofzumahaus A, Holland F, Bohn B, Brauers T, Fuchs H, Hu M, Häseler R, Kita K, Kondo Y (2013) Missing OH source in a suburban environment near Beijing: observed and modelled OH and HO2 concentrations in summer 2006. Atmos Chem Phys 13(2):1057–1080
Modica AP (1966) Kinetics and equilibria of the difluorocarbene radical decomposition behind shock waves. J Chem Phys 44(4):1585–1589
Moon DJ, Chung MJ, Kim H, Kwon YS, Ahn BS (2002) Pyrolysis of trifluoromethane to produce hexafluoropropylene. Ind Eng Chem Res 41(12):2895–2902
Ni Z (2008) Review and prospect of fluorochemical industry in China. Organo Fluor Ind 2:23–26 (in Chinese)
Russell MH, Hoogeweg G, Webster EM, Ellis DA, Waterland RL, Hoke RA (2012) TFA from HFO-1234yf: accumulation and aquatic risk in terminal water bodies. Environ Toxicol Chem 31(9):1957–1965
Schwarzbach SE (1995) CFC alternatives under a cloud. Nature 376(6538):297–298
Scott BF, Mactavish D, Spencer C, Strachan WMJ, Muir DCG (2000) Haloacetic acids in Canadian lake waters and precipitation. Environ Sci Technol 34(20):4266–4272
Scott BF, Spencer C, Martin JW, Barra R, Bootsma HA, Jones KC, Johnston AE, Muir DC (2005) Comparison of haloacetic acids in the environment of the Northern and Southern Hemispheres. Environ Sci Technol 39(22):8664–8670
Scott BF, Spencer C, Mabury SA, Muir DC (2006) Poly and perfluorinated carboxylates in North American precipitation. Environ Sci Technol 40(23):7167–7174
Taniyasu S, Kannan K, Yeung LWY, Kwok KY, Lam PKS, Yamashita N (2008) Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2-C4) in precipitation by liquid chromatography–tandem mass spectrometry: comparison to patterns of long-chain perfluorinated acids (C5-C18). Anal Chim Acta 619(2):221–230
Tschuikow-Roux E, Marte JE (1965) Thermal decomposition of fluoroform in a single-pulse shock tube. J Chem Phys 43(4):1438–1438
UNFCCC (2006) AM0001: Incineration of HFC 23 waste streams.
US EPA (1992) Framework for ecological risk assessment. Risk Assessment Forum Washington, DC
Visscher PT, Culbertson CW, Oremland RS (1994) Degradation of trifluoroacetate in oxic and anoxic sediments. Nature 369(6483):729–731
Wallington TJ, Schneider WF, Worsnop DR, Nielsen OJ, Sehested J, Debruyn WJ, Shorter JA (1994) The environmental impact of CFC replacements HFCs and HCFCs. Environ Sci Technol 28(7):320A–326A
Wu J, Martin JW, Zhai Z, Lu K, Li L, Fang X, Jin H, Hu J, Zhang J (2014) Airborne trifluoroacetic acid and its fraction from the degradation of HFC-134a in Beijing, China. Environ Sci Technol 48(7):3675–3681
Xiang X, Zheng G, Shen Z, Wu P (2009) Multiphase mass transfer model on the accumulation of TFA in regional environments. Res Environ Sci 22(9):1108–1112 (in Chinese)
Yang J, Liu Z, Feng L (2009) Base Ecological risk of PAHs in water of Yangtze River Estuary. Res Environ Sci 22(7):784–787 (in Chinese)
Zehavi D, Seiber JN (1996) An analytical method for trifluoroacetic acid in water and air samples using headspace gas chromatographic determination of the methyl ester. Anal Chem 68(19):3450–3459
Zhai Z, Wu J, Hu X, Li L, Guo J, Zhang B, Hu J, Zhang J (2015) A 17-fold increase of trifluoroacetic acid in landscape waters of Beijing, China during the last decade. Chemosphere 129:110–117
Zhang Y, Lin Y, Sun Q (2002) Ecological risk assessment of hazardous waste. China Environmental Science Press, Beijing (in Chinese)
Zhang B, Zhai Z, Zhang J (2018) Distribution of trifluoroacetic acid in gas and particulate phases in Beijing from 2013 to 2016. Sci Total Environ 634:471–477
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 41575140 and No. 41775143).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. TFA in the environments surrounding fluorochemical production plants were measured.
2. Regional TFA pollution exists in the areas near fluorochemical industrial activity.
3. High TFA concentration may be related to the fluorochemical production process associated with high-temperature pyrolysis.
4. TFA did cause potential damage to the aquatic ecosystem of Jinan.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Xie, G., Cui, J., Zhai, Z. et al. Distribution characteristics of trifluoroacetic acid in the environments surrounding fluorochemical production plants in Jinan, China. Environ Sci Pollut Res 27, 983–991 (2020). https://doi.org/10.1007/s11356-019-06689-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06689-4