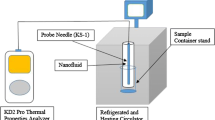
Abstract
CO2 and H2S removal from flue gases is indispensable to be done for protection of environment with respect to global warming as well as clean air. Chemical absorption is one of the most developed and capable techniques for the removal of these sour gases. Among the many solvents, ionic liquids (ILs) are more capable due to their desirable green solvent properties. However, ILs being usually costlier, the blends of ILs and amines are more suggestive for absorption. In the present work, various essential characterization properties such as density, viscosity, sound velocity, and refractive index of two ionic liquid–amine blend systems viz. (1) 2-Hydroxy ethyl ammonium formate (HEF) + 1-(2-aminoethyl) piperazine (AEP) and (2) 2-Hydroxy ethyl ammonium formate (HEF) + 2-Amino-2-methyl-1-propanol (AMP) are reported. The temperature range for which all the measurements were conducted is 298.15 to 333.15 K. For both systems of (HEF + AEP) and (HEF + AMP), HEF mass fractions were varied from 0.2 to 0.8.The density and viscosity results were correlated as a function of temperature and concentration of ionic liquid and amine with Redlich-Kister and Grunberg-Nissan models, respectively. Moreover, feed forward neural network model (ANN) is explored for correlating experimentally determined sound velocity and refractive index data. The measured properties are further analyzed to estimate various thermodynamic as well as transport properties such as diffusivity of CO2/H2S in the (HEF + AEP) and (HEF + AMP), thermal expansion coefficients, and isentropic compressibility, ΔG0, ΔS0, ΔH0, using the available models in the literature.









Similar content being viewed by others
References
Alvarez KEA, Gómez-Díaz D, Rubia MDL, Navaza JM (2006) Densities and viscosities of aqueous ternary mixtures of 2-(methylamino) ethanol and 2-(ethylamino)ethanol with diethanolamine, triethanolamine, N methyldiethanolamine, or 2-amino-1-methyl-1-propanol from 298.15 to 323.15. J Chem Eng Data 51:955–962
Alvarez E, Fernando C, Diego G, Jose MN (2010) Density, speed of sound, isentropic compressibility, and excess volume of binary mixtures of 1-amino-2-propanol or 3-amino-1-propanol with 2-amino-2-methyl-1-propanol, diethanolamine, or triethanolamine from (293.15to 323.15) K. J Chem Eng Data 55:2567–2575
Anastas PT, Warner JC (1988) Green chemistry: theory and practice, Oxford. University Press, New York, p 30
Andre P, Keng LA, Kenneth NM, Shusheng P (2011) Density, viscosity and electrical conductivity of protic alkanolammonium ionic liquids. Phys Chem 13:5136–5143
Aronu UE, Gondal S, Hessen ET, Haug-Warberg T, Hartono A, Hoff KA, Svendsen HF (2011) Solubility of CO2 in 15, 30, 45 and 60 mass% MEA from 40 to 120 °C and model representation using the extended UNIQUAC framework. Chem Eng Sci 66:6393–6406
Braun ON, Persson UA, Karlsson HT (2001) Densities and viscosities of mono (ethylene glycol) + 2-amino-2-methyl-1-propanol + Water. J Chem Eng Data 46:805–808
Choi J, Kim Y, Nam S, Yun S, Yoon Y, Lee J (2016) CO2 absorption characteristics of a piperazine derivative with primary, secondary, and tertiary amino groups. Kor J Chem Eng 33:3222–3230
Cota I, Gonzalez-Olmos R, Iglesias M, Medina F (2007) New short aliphatic chain ionic liquids: synthesis, physical properties, and catalytic activity in aldol condensations. J Phys Chem B 111:12468–12477
Das B, Deogam B, Mandal BP (2017) Experimental and theoretical studies on efficient carbon dioxide capture using novel bis (3-amino propyl) amine (APA)-activated aqueous 2-amino-2-methyl-1-propanol (AMP) solutions. RSC Adv 7:21518–21530
García-Abuín A, Gomez-Díaz D, Rubia MD, Navaza JM (2011) Density, speed of sound, viscosity, refractive index, and excess volume of N-methyl-2-pyrrolidone + ethanol (or water or ethanolamine) from T = (293.15 to 323.15) K. J Chem Eng Data 56:646–651
Ghani NA, Sairi NA, Aroua MK, Alias Y (2014) Density, surface tension, and viscosity of ionic liquids (1-ethyl-3-methylimidazolium diethylphosphate and 1,3-dimethylimidazolium dimethyl phosphate) aqueous ternary mixtures with MDEA. J Chem Eng Data 59:1737–1746
Gokul CK, Bradley DF, Bani HC, Alexander IN, Srinivasa RR (2005) Viscosity increase with temperature in cationic surfactant solutions due to the growth of wormlike micelles. Langmuir 21:10998–11004
Guo H, Zhou Z, Jing G (2013) Kinetics of carbon dioxide absorption into aqueous [Hmim] [Gly] solution. Int J Greenhouse Gas Control 16:197–205
Hamzehie ME, Najibi H (2015) Prediction of carbon dioxide loading capacity in amino acid salt solutions as new absorbents using artificial neural network and Deshmukhe Mather models. J Nat Gas Sci Eng 27:676–685
Hamzehie ME, Fattahi M, Najibi H, Bruggen BVD, Mazinani S (2015) Application of artificial neural networks for estimation of solubility of acid gases (H2S and CO2) in 32 commonly ionic liquid and amine solutions. J Nat Gas Sci Eng 24:106–114
Heintz YJ, Sehabiague L, Morsi BI, Jones KL, Luebke DR, Pennline HW (2009) Hydrogen sulfide and carbon dioxide removal from dry fuel gas streams using an ionic liquid as a physical solvent. Energy Fuel 23:4822–4830
Jalili AH, Mehdizadeh A, Shokouhi M, Ahmadi AN, Hosseini-Jenab M (2010a) Fatem-inassab F, 2010a. Solubility and diffusion of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. J Chem Thermodyn 42:1298–1303
Jalili AH, Mehdizadeh A, Shokouhi M, Sakhaeinia H, Taghikhani V (2010b) Solubility of CO2 in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions. J Chem Thermodyn 42:787–791
John G, Nandhibatla VS (2004) Densities, viscosities, speeds of sound, and relative permittivities for water + cyclic amides (2-pyrrolidinone, 1-methyl-2-pyrrolidinone, and 1-vinyl-2-pyrrolidinone) at different temperatures. J Chem Eng Data 49:235–242
Kristian OSH, Kurt VM, Thomas MN, Per-Olof AS (2005) Refractive index of liquid water in different solvent models. J Phys Chem A 109:905–914
Kuan H, Jian FL, You TW, Xing BH, Zhi BZ (2013) Absorption of SO2 in aqueous solutions of mixed hydroxyl ammonium dicarboxylate ionic liquids. Chem Eng J 215–216:36–44
Kurnia KA, Harris F, Wilfred CD, Mutalib MIA, Murugesan T (2009) Thermodynamic properties of CO2 absorption in hydroxyl ammonium ionic liquids at pressures of (100–1600) kPa. J Chem Thermodyn 41:1069–1073
Li L, Zhang J, Li Q, Guo B, Zhao T, Sha F (2014) Density, viscosity, surface tension, and spectroscopic properties for binary system of 1, 2-ethanediamine + diethylene glycol. Thermochim Acta 590:91–99
Mahinder R, Theo WL, Thijs JHV (2012) State-of-the-Art of CO2 capture with ionic liquids. Ind Eng Chem Res 51(24):8149–8177
Mandal BP, Kundu M, Bandhyopadhyay SS (2003) Density and viscosity of aqueous solutions of (N-methyldiethanolamine + monoethanolamine), (N-methyldiethanolamine + diethanolamine), (2-amino-2-methyl-1-propanol + monoethanolamine) and (2-Amino-2-methyl-1-propanol + diethanolamine). J Chem Eng Data 48:703–707
Mandal BP, Kundu M, Padhiyar NU, Bandyopadhyay SS (2004a) Physical solubility and diffusivity of N2O and CO2 into aqueous solutions of (2-amino-2-methyl-1-propanol + diethanolamine) and (N-methyldiethanolamine + diethanolamine). J Chem Eng Data 49:264–270
Mandal BP, Biswas AK, Bandyopadhyay SS (2004b) Selective absorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Sep Purif Technol 35:191–202
McCoy CA, Gregor MC, Polsin DN, Fratanduono DE, Celliers PM, Boehly TR, Meyerhofer DD (2016) Measurements of the sound velocity of shock-compressed liquid silicato1100GPa. J Appl Phys 120:235901
Nithiyanantham S (2019) Ultrasonic velocity models in liquids (micro- and nanofluids): theoretical validations. Int Nano Lett 9(2):89–97
Noman H, Orville CS (1987) Absorption of H2S into aqueous methyldiethanolamine. Chem Eng Commun 59:85–93
Noman H, Orvlile CS (1984) Molecular diffusivity of hydrogen sulphide in water. J Chem Eng Data 29:20–22
Nwaoha C, Odoh K, Ikpatt E, Orji R, Idem R (2017) Process simulation, parametric sensitivity analysis and ANFIS modeling of CO2 capture from natural gas using aqueous MDEA–PZ blend solution. J Environ Chem Eng 5:5588–5598
Pani F, Gaunand A, Richon D, Cadours R, Bouallou C (1997) Absorption of H2S by an aqueous methyldiethanolamine solution at 296 and 343 K. J Chem Eng Data 42:865–870
Patil GN, Vaidya PD, Kenig EY (2012) Reaction kinetics of CO2 in aqueous methyl- and dimethyl monoethanolamine solutions. Ind Eng Chem Res 51:1592–1600
Paul S, Mandal BP (2006) Density and viscosity of aqueous solutions of 2-piperidineethanol, (2-piperidinethanol + monoethanolamine), and (2-piperidinethanol + diethanolamine) from (288 to 333) K. J Chem Eng Data 51:1406–1410
Paul S, Ghoshal AK, Mandal B (2009) Kinetics of absorption of carbon dioxide into aqueous blends of 2-(1-piperazinyl)-ethylamine and N-methyldiethanolamine. Chem Eng Sci 64:1618–1622
Pinto DD, Monteiro JGM, Johnsen B, Svendsen HF, Knuutila H (2014) Density measurements and modeling of loaded and unloaded aqueous solutions of MDEA (N-methyldiethanolamine), DMEA (N, N-dimethylethanolmine), DEEA (diethylethanolamine) and MAPA (N-methyl-1, 3-diaminopropane). Int J Greenhouse Gas Control 25:173–185
Rascol E, Meyer M, Huor MH, Prevost M (1997) Modelisation and simulation of the absorption of CO2 and H2S into mixed alkanolamine solutions. Hung J Ind Chem 25:11–16
Remi B, Didier M (2018) Improved model for the refractive index: application to potential components of ambient aerosol. Phys Chem Chem Phys 20:22017–22026
Rinker EB, Ashour SS, Sandall OC (2000) Absorption of carbon dioxide into aqueous blends of diethanolamine and methyldiethanolamine. Ind Eng Chem Res 39:4346–4356
Saha AK, Bandyopadhyay SS, Saju P, Biswas AK (1993) Selective removal of H2S from gases containing H2S and CO2 by absorption into aqueous-solutions of 2-amino-2-methyl-1-propanol. Ind Eng Chem Res 32:3051–3055
Samuel S, Aruni MD, Han X, Joan FB (2015) Effect of cation on physical properties and CO2 solubility for phosphonium-based ionic liquids with 2-cyanopyrrolide anions. J Phys Chem B 119(35):11807–11814
Satish K, Jae HCM (2014) Ionic liquid-amine blends and CO2 BOLs: prospective solvents for natural gas sweetening and CO2 capture technology—a review. Int J Greenhouse Gas Control 20:87–116
Sedghamiz MA, Rasoolzadeh A, Rahimpour MR (2015) The ability of artificial neural network in prediction of the acid gases solubility in different ionic liquids. J CO2 Util 9:39–47
Sergey L, Anna P, Magdalena KO (2003) Sound velocity and acoustic nonlinearity parameter for fluids. Thermodynamic premises. arXiv:physics/0303117
Shaikh MS, Shariff AM, Bustam MA, Murshid G (2013) Physical properties of aqueous blends of sodium glycinate (SG) and piperazine (PZ) as a solvent for CO2 capture. J Chem Eng Data 58:634–638
Shokouhi M, Adibi M, Jalili AH, Hosseini-Jenab M, Mehdizadeh A (2010) Solubility and diffusion of H2S and CO2 in the ionic liquid 1-(2-hydroxyethyl)-3-methylimidazolium tetrafluoroborate. J Chem Eng Data 55:1663–1668
Versteeg GF, Swaalj WV (1988) Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkaloamines solutions. J Chem Eng Data 33:29–34
Wu S-T (1991) A semi empirical model for liquid-crystal refractive index dispersions. J Appl Phys 69(4):2080–2087
Xiao LY, Suo JZ, Xing ML (2007) Hydroxyl ammonium ionic liquids: synthesis, properties, and solubility of SO2. J Chem Eng Data 52:596–599
Xiao LY, Xiao YC, Rong XQ, Bao YP, Yi W, Min H, Jin QL, Jing YZ, Geng GL (2019) Enhanced CO2 capture by reducing cation–anion interactions in hydroxyl-pyridine anion-based ionic liquids. Dalton Trans 48:2300–2307
Yang ZY, Soriano AN, Caparanga AR, Li MH (2010) Equilibrium solubility of carbon dioxide in (2-amino-2-methyl-1-propanol + piperazine + water). J Chem Thermodynamics 42:659–665
Yuan X, Zhang S, Liu J, Lua X (2007) Solubilities of CO2 in hydroxyl ammonium ionic liquids at elevated pressures. Fluid Phase Equilib 257:195–200
Zhao Y, Zhang X, Zeng S, Zhou Q, Dong H, Tian X, Zhang S (2010) Density, viscosity, and performances of carbon dioxide capture in 16 absorbents of amine + ionic liquid + H2O, ionic liquid + H2O, and amine + H2O systems. J Chem Eng Data 55:3513–3519
Zhou Q, Wu Y, Chan CW, Tontiwachwuthikul P (2011) From neural network to neuro-fuzzy modeling: applications to the carbon dioxide capture process. Energy Procedia 4:2066–2073
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 163 kb)
Rights and permissions
About this article
Cite this article
Balchandani, S., Mandal, B., Dharaskar, S. et al. Thermally induced characterization and modeling of physicochemical, acoustic, rheological, and thermodynamic properties of novel blends of (HEF + AEP) and (HEF + AMP) for CO2/H2S absorption. Environ Sci Pollut Res 26, 32209–32223 (2019). https://doi.org/10.1007/s11356-019-06305-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06305-5