Abstract
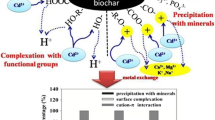
To clarify the adsorption mechanism of multi-ions on biochars in competitive environment is very important for the decontamination of co-existed heavy metals. Herein, tobacco stem was pyrolyzed in different temperatures with selected residences to obtain biochars with various surface chemistry. Then the adsorption of co-existed typical heavy-metal ions like lead, cadmium, and copper was studied, followed with systematic analysis of surface properties of the post-adsorption biochars. After carefully examining the adsorption performance and surface property alteration of the demineralized biochars, the adsorption mechanism of multi-ions in competitive environment was discovered. Lead showed the most competitive nature with co-existence of cadmium and copper, but the adsorption capacity reduced significantly with the removal of minerals. Combined with the observation of large amount of lead containing crystals on the post-adsorption biochars, the main adsorption mechanism of lead should be precipitation. The adsorb capability of copper barely changed for biochars with and without minerals, which suggests the best affinity of copper on surface functional groups even with large content of competitors. Biochar that pyrolyzed in 700 °C for 6 h that contained more aromatic structures showed the highest sorbing capability of cadmium, which suggested the dominant position of cation-π interaction in cadmium removal.








Similar content being viewed by others
References
Andersen FA, Brečević L, Beuter G, Dell’Amico DB, Calderazzo F, Bjerrum NJ, Underhill AE (1991) Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem Scand 45:1018–1024
Bayramoğlu G, Arıca MY (2008) Removal of heavy mercury(II), cadmium(II) and zinc(II) metal ions by live and heat inactivated Lentinus edodes pellets. Chem Eng J 143:133–140
Beruto D, Barco L, Searcy AW, Spinolo G (2010) Characterization of the porous CaO particles formed by decomposition of CaCO3 and Ca(OH)2 in vacuum. J Am Ceram Soc 63:439–443
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Cheng Q, Huang Q, Khan S, Liu Y, Liao Z, Li G, Ok YS (2016) Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol Eng 87:240–245
Deng J, Liu Y, Liu S, Zeng G, Tan X, Huang B, Tang X, Wang S, Hua Q, Yan Z (2017) Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci 506:355–364
Elwakeel KZ, Atia AA, Guibal E (2014) Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour Technol 160:107–114
Goertzen SL, Thériault KD, Oickle AM, Tarasuk AC, Andreas HA (2010) Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 48:1252–1261
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Kasselouri V, Dimopoulos G, Parissakis G (1995) Decomposition of CaCO3 in the presence of organic acids. Cem Concr Res 25:955–960
Keiluweit M, Kleber M (2009) Molecular-level interactions in soils and sediments: the role of aromatic π-systems. Environ Sci Technol 43:3421–3429
Kılıç M, Kırbıyık Ç, Çepelioğullar Ö, Pütün AE (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862
Li M, Liu Q, Guo L, Zhang Y, Lou Z, Wang Y, Qian G (2013) Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour Technol 141:83–88
Li W, Zhang L-B, Peng J-H, Li N, Zhu X-y (2008) Preparation of high surface area activated carbons from tobacco stems with K2CO3 activation using microwave radiation. Ind Crop Prod 27:341–347
Lin Y, Yan W, Sheng K (2016) Effect of pyrolysis conditions on the characteristics of biochar produced from a tobacco stem. Waste Manag Res 34:793–801
Ma JC, Dougherty DA (1997) The cation−π interaction. Chem Rev 97:1303–1324
Mandal S, Sarkar B, Igalavithana AD, Ok YS, Yang X, Lombi E, Bolan N (2017) Mechanistic insights of 2,4-D sorption onto biochar: influence of feedstock materials and biochar properties. Bioresour Technol 246:160–167
Meng A, Zhang Y, Zhuo J, Li Q, Qin L (2015) Investigation on pyrolysis and carbonization of Eupatorium adenophorum Spreng and tobacco stem. J Energy Inst 88:480–489
Oickle AM, Goertzen SL, Hopper KR, Abdalla YO, Andreas HA (2010) Standardization of the Boehm titration: part II. Method of agitation, effect of filtering and dilute titrant. Carbon 48:3313–3322
Park J-H, Cho J-S, Ok YS, Kim S-H, Kang S-W, Choi I-W, Heo J-S, DeLaune RD, Seo D-C (2015) Competitive adsorption and selectivity sequence of heavy metals by chicken bone-derived biochar: batch and column experiment. J Environ Sci Health A 50:1194–1204
Park J-H, Cho J-S, Ok YS, Kim S-H, Heo J-S, Delaune RD, Seo D-C (2016a) Comparison of single and competitive metal adsorption by pepper stem biochar. Arch Agron Soil Sci 62:617–632
Park J-H, Ok YS, Kim S-H, Cho J-S, Heo J-S, Delaune RD, Seo D-C (2016b) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Peng H, Gao P, Chu G, Pan B, Peng J, Xing B (2017) Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Puziy AM, Poddubnaya OI, Martínez-Alonso A, Suárez-García F, Tascón JMD (2002) Synthetic carbons activated with phosphoric acid: I. Surface Chem Ion Bind Properties Carbon 40:1493–1505
Qin Z, Luo X, Rong N, Wang M, Wang J, Wu J, Li Z, Duns GJ, He F, Chen H, Yang L (2016) Preparation and analysis of physicochemical properties of tobacco stem biochar. J Nanosci Nanotechnol 16:12237–12243
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem Eur J 15:4195–4203
Shen Z, Hou D, Zhao B, Xu W, Ok YS, Bolan NS, Alessi DS (2018) Stability of heavy metals in soil washing residue with and without biochar addition under accelerated ageing. Sci Total Environ 619-620:185–193
Suliman W, Harsh JB, Abu-Lail NI, Fortuna AM, Dallmeyer I, Garcia-Perez M (2016) Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 84:37–48
Sun J, He F, Pan Y, Zhang Z (2017) Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 67:12–22
Sun XM, Li YD (2004) Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angewandte Chemie-International Edition 43:597–601
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Teng J, Zeng X, Xu X, Yu J-G (2018) Assembly of a novel porous 3D graphene oxide-starch architecture by a facile hydrothermal method and its adsorption properties toward metal ions. Mater Lett 214:31–33
Uchimiya M, Lima IM, Thomas Klasson K, Chang S, Wartelle LH, Rodgers JE (2010) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agric Food Chem 58:5538–5544
Vithanage M, Rajapaksha AU, Ahmad M, Uchimiya M, Dou X, Alessi DS, Ok YS (2015) Mechanisms of antimony adsorption onto soybean stover-derived biochar in aqueous solutions. J Environ Manag 151:443–449
Wang F, Sun H, Ren X, Liu Y, Zhu H, Zhang P, Ren C (2017) Effects of humic acid and heavy metals on the sorption of polar and apolar organic pollutants onto biochars. Environ Pollut 231:229–236
Wang Z, Liu G, Zheng H, Li F, Ngo HH, Guo W, Liu C, Chen L, Xing B (2015) Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour Technol 177:308–317
Xu X, Jiang X-Y, Jiao F-P, Chen X-Q, Yu J-G (2018) Tunable assembly of porous three-dimensional graphene oxide-corn zein composites with strong mechanical properties for adsorption of rare earth elements. J Taiwan Inst Chem Eng 85:106–114
Yin S, Wu Y, Xu W, Li Y, Shen Z, Feng C (2016) Contribution of the upper river, the estuarine region, and the adjacent sea to the heavy metal pollution in the Yangtze Estuary. Chemosphere 155:564–572
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhang C, Shan B, Tang W, Zhu Y (2017a) Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour Technol 238:352–360
Zhang H, Chen C, Gray EM, Boyd SE (2017b) Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 105:136–146
Zheng H, Wang Z, Zhao J, Herbert S, Xing B (2013) Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ Pollut 181:60–67
Zhou L, Liu Y, Liu S, Yin Y, Zeng G, Tan X, Hu X, Hu X, Jiang L, Ding Y, Liu S, Huang X (2016) Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol 218:351–359
Zhou N, Chen H, Xi J, Yao D, Zhou Z, Tian Y, Lu X (2017) Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour Technol 232:204–210
Zhou Z, Xu Z, Feng Q, Yao D, Yu J, Wang D, Lv S, Liu Y, Zhou N, M-e Z (2018) Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. J Clean Prod 187:996–1005
Acknowledgments
The authors also would like to thank Mr. Deming Liu (Analysis Center, Hunan Agricultural University) for his help in performing the ICP-MS analysis.
Funding
The work was financial supported by the National Natural Science Foundation of China (Nos. 51703061, 21706060, 51504212), the Natural Science Foundation of Hunan Province (No. 2018JJ3214), the Research Foundation of Education Department of Hunan Province (No. 18A103), and the Fujian Provincial Key Laboratory of Functional Materials and Applications (Xiamen University of Technology, No. fma2017202).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Responsible editor: Tito Roberto Cadaval
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 6.11 mb)
Rights and permissions
About this article
Cite this article
Zhou, N., Zu, J., Feng, Q. et al. Effect of pyrolysis condition on the adsorption mechanism of heavy metals on tobacco stem biochar in competitive mode. Environ Sci Pollut Res 26, 26947–26962 (2019). https://doi.org/10.1007/s11356-019-05917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05917-1