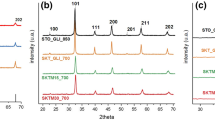
Abstract
Lanthanide perovskite catalysts doped with limited palladium (to improve activity) and cerium (to improve sulfur resistance) were prepared using sol–gel method. In different B sites, lanthanide perovskites were studied at harsh conditions for H2 selective catalytic reduction of NO. The activity sequence was as follows: LaCeMnPd > LaCeCoPd > LaCeFePd. LaCeMnPd had a high NO conversion of 96.6% at only 150 °C. And it also had a surprising SO2 resistance in different SO2 concentrations. After cutting out SO2, NO conversion recovered rapidly to its original level, indicating that the slight deactivation was reversible. In addition, the effect of gas hourly space velocity, H2/NO ratio, O2, and SO2 concentration was studied. And XRD, energy-dispersive X-ray, SEM, XPS, H2-temperature programmed reduction, and NH3-temperature programmed desorption were performed to characterize the catalysts.

ᅟ





Similar content being viewed by others
References
Acke F, Westerberg B, Skoglundh M (1998) Selective reduction of NO by HNCO over Pt promoted Al2O3. J Catal 179:528–536
Ahn K, Kim H, Chung Y-C, Kim H-R, Son J-W, Lee H-W, Lee J-H (2010) Effects of B-site substitution on the surface adsorption properties and catalytic activities of La0.8Sr0.2(Mn1−xCox)O3. Appl Catal A Gen 387:203–208
Arendt E, Maione A, Klisinska A, Sanz O, Montes M, Suarez S, Blanco J, Ruiz P (2008) Structuration of LaMnO3 perovskite catalysts on ceramic and metallic monoliths: Physico-chemical characterisation and catalytic activity in methane combustion. Appl Catal A Gen 339:1–14
Barrera A, Viniegra M, Bosch P, Lara VH, Fuentes S (2001) Pd/Al2O3-La2O3 catalysts prepared by sol–gel characterization and catalytic activity in the NO reduction by H2. Appl Catal B Environ 34:97–111
Benrabaa R, Löfberg A, Rubbens A, Bordes-Richard E, Vannier RN, Barama A (2013) Structure, reactivity and catalytic properties of nanoparticles of nickel ferrite in the dry reforming of methane. Catal Today 203:188–195
Chen JP, Yang RT (1990) Mechanism of poisoning of the V2O5/TiO2 catalyst for the reduction of NO by NH3. J Catal 125:411–420
Chen J, Shen M, Wang X, Qi G, Wang J, Li W (2013) The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl Catal B Environ 134-135:251–257
Costa CN, Efstathiou AM (2007) Mechanistic aspects of the H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst. J Phys Chem C 111:3010–3020
Das S, Roychoudhury P, De S, Roy A, Chatterjee S, De K (2018) Magnetic and electrical transport of the cation-deficient LaMnO3: common origin for both Sr-doping and self-doping effects. Phys B Condens Matter 544:17–22
Elemans JBAA, Van Laar B, Van Der Veen KR, Loopstra BO (1971) The crystallographic and magnetic structures of La1−xBaxMn1−xMexO3 (Me = Mn or Ti). J Solid State Chem 3:238–242
Fan J, Wu X, Yang L, Weng D (2007) The SMSI between supported platinum and CeO2–ZrO2–La2O3 mixed oxides in oxidative atmosphere. Catal Today 126:303–312
Fierro JLG, Tascón JMD, Tejuca LG (1984) Physicochemical properties of LaMnO3: reducibility and kinetics of O2 adsorption. J Catal 89:209–216
Forzatti P (2001) Present status and perspectives in de-NOx SCR catalysis. Appl Catal A Gen 222:221–236
Giebeler L, Kießling D, Wendt G (2007) LaMnO3 perovskite supported noble metal catalysts for the total oxidation of methane. Chem Eng Technol 30:889–894
Giraudon JM, Elhachimi A, Wyrwalski F, Siffert S, Aboukaïs A, Lamonier JF, Leclercq G (2007) Studies of the activation process over Pd perovskite-type oxides used for catalytic oxidation of toluene. Appl Catal B Environ 75:157–166
Gomez-Garcia MA, Pitchon V, Kiennemann A (2005) Pollution by nitrogen oxides: an approach to NOx abatement by using sorbing catalytic materials. Environ Int 31:445–467
González Tejuca L, Bell AT, Fierro JLG, Peña MA (1988) Surface behaviour of reduced LaCoO3 as studied by TPD of CO, CO2 and H2 probes and by XPS. Appl Surf Sci 31:301–316
Hosseini SA, Mehri B, Niaei A, Izadkhah B, Alvarez-Galvan C, Fierro JGL (2018) Selective catalytic reduction of NOx by CO over LaMnO3 nano perovskites prepared by microwave and ultrasound assisted sol–gel method. J Sol-Gel Sci Technol 85:1–10
Huilina L, Guangboa Z, Rushana B, Yongjina C, Gidaspow D (2000) A coal combustion model for circulating fluidized bed boilers. Fuel 79:165–172
Imran M, Kim DH, Al-Masry WA, Mahmood A, Hassan A, Haider S, Ramay SM (2013) Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis. Polym Degrad Stab 98:904–915
Jin R, Liu Y, Wang Y, Cen W, Wu Z, Wang H, Weng X (2014) The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl Catal B Environ 148-149:582–588
Kang M, Park ED, Kim JM, Yie JE (2006) Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal Today 111:236–241
Koponen MJ, Suvanto M, Pakkanen TA, Kallinen K, Kinnunen T-JJ, Härkönen M (2005) Synthetic studies of ABB′O3 (A=La, Pr, Nd; B=Fe,Mn; B=Pd, Pt) perovskites. Solid State Sci 7:7–12
Kucharczyk B, Tylus W (2007) Metallic monolith supported LaMnO3 perovskite-based catalysts in methane combustion. Catal Lett 115:122–132
Lam DJ, Veal BW, Ellis DE (1980) Electronic structure of lanthanum perovskites with 3d transition elements. Phys Rev B 22:5730–5739
Lee YN, Lago RM, Fierro JLG, González J (2001) Hydrogen peroxide decomposition over Ln1−xAxMnO3 (Ln = La or Nd and A = K or Sr) perovskites. Appl Catal A Gen 215:245–256
Ling F, Anthony OC, Xiong Q, Luo M, Pan X, Jia L, Huang J, Sun D, Li Q (2016) PdO/LaCoO3 heterojunction photocatalysts for highly hydrogen production from formaldehyde aqueous solution under visible light. Int J Hydrog Energy 41:6115–6122
Lisi L, Bagnasco G, Ciambelli P, Rossi SD, Porta P, Russo G, Turco M (1999) Perovskite-type oxides. II. Redox properties of LaMn1-xCuxO3 and LaCo1-xCuxO3 and methane catalytic combustion. J Solid State Chem 146:176–183
Liu Z, Li J, Junaid ASM (2010) Knowledge and know-how in improving the sulfur tolerance of deNOx catalysts. Catal Today 153:95–102
Liu D, Yuan P, Liu H, Cai J, Qin Z, Tan D, Zhou Q, He H, Zhu J (2011) Influence of heating on the solid acidity of montmorillonite: a combined study by DRIFT and Hammett indicators. Appl Clay Sci 52:358–363
Liu Z, Li J, Woo SI (2012) Recent advances in the selective catalytic reduction of NOx by hydrogen in the presence of oxygen. Energy Environ Sci 5:8799
Liu D, Yuan P, Liu H, Cai J, Tan D, He H, Zhu J, Chen T (2013) Quantitative characterization of the solid acidity of montmorillonite using combined FTIR and TPD based on the NH3 adsorption system. Appl Clay Sci 80-81:407–412
Miedziak PJ, Tang Z, Davies TE, Enache DI, Bartley JK, Carley AF, Herzing AA, Kiely CJ, Taylor SH, Hutchings GJ (2009) Ceria prepared using supercritical antisolvent precipitation: a green support for gold–palladium nanoparticles for the selective catalytic oxidation of alcohols. J Mater Chem 19:8619
Natile MM, Ugel E, Maccato C, Glisenti A (2007) LaCoO3: effect of synthesis conditions on properties and reactivity. Appl Catal B Environ 72:351–362
Nishihata Y, Mizuki J, Akao T, Tanaka H, Uenishi M, Kimura M, Okamoto T, Hamada N (2002) Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418:164–167
Pan S, Luo H, Li L, Wei Z, Huang B (2013) H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J Mol Catal A Chem 377:154–161
Park TS, Jeong SK, Hong SH, Hong SC (2001) Selective catalytic reduction of nitrogen oxides with NH3 over natural manganese ore at low temperature. Ind Eng Chem Res 40:44915
Petunchi JO, Hall WK (1993) On the role of nitrogen dioxide in the mechanism of the selective reduction of NOx over Cu-ZSM-5 zeolite. Appl Catal B Environ 2:L17–L26
Ponce S, Peña MA, Fierro JLG (2000) Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl Catal B Environ 24:193–205
Radojevic M (1998) Reduction of nitrogen oxides in flue gases. Environ Pollut 102:685–689
Reddy CR, Bhat YS, Nagendrappa G, Jai Prakash BS (2009) Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal Today 141:157–160
Rojas ML, Fierro JLG, Tejuca LG, Bell AT (1990) Preparation and characterization of LaMn1−xCuxO3+λ perovskite oxides. J Catal 124:41–51
Royer S, Alamdari H, Duprez D, Kaliaguine S (2005) Oxygen storage capacity of La1−xA′xBO3 perovskites (with A′=Sr, Ce; B=Co, Mn)—relation with catalytic activity in the CH4 oxidation reaction. Appl Catal B Environ 58:273–288
Savva P, Efstathiou A (2008) The influence of reaction temperature on the chemical structure and surface concentration of active NOx in H2-SCR over Pt/MgO-CeO2: SSITKA-DRIFTS and transient mass spectrometry studies. J Catal 257:324–333
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst 32:751–767
Shi H, Li X, Xia J, Lu X, Zuo S, Luo S, Yao C (2017) Sol–gel synthesis of LaBO 3/attapulgite (B=Mn, Fe, Co, Ni) nanocomposite for NH 3 -SCR of NO at low temperature. J Inorg Organomet Polym Mater:1–7
Skalska K, Miller JS, Ledakowicz S (2010) Trends in NOx abatement: a review. Sci Total Environ 408:3976–3989
Tanaka H, Misono M (2001) Advances in designing perovskite catalysts. Curr Opinion Solid State Mater Sci 5:381–387
Tanaka H, Tan I, Uenishi M, Taniguchi M, Kimura M, Nishihata Y, Mizuki J (2006a) LaFePdO3 perovskite automotive catalyst having a self-regenerative function. J Alloys Compd 408-412:1071–1077
Tanaka H, Uenishi M, Taniguchi M, Tan I, Narita K, Kimura M, Kaneko K, Nishihata Y, Mizuki J (2006b) The intelligent catalyst having the self-regenerative function of Pd, Rh and Pt for automotive emissions control. Catal Today 117:321–328
Tejuca LG, Fierro JLG (1989) XPS and TPD probe techniques for the study of LaNiO3 perovskite oxide. Thermochim Acta 147:361–375
Tzimpilis E, Moschoudis N, Stoukides M, Bekiaroglou P (2008) Preparation, active phase composition and Pd content of perovskite-type oxides. Appl Catal B Environ 84:607–615
Uenishi M, Taniguchi M, Tanaka H, Kimura M, Nishihata Y, Mizuki J, Kobayashi T (2005) Redox behavior of palladium at start-up in the perovskite-type LaFePdOx automotive catalysts showing a self-regenerative function. Appl Catal B Environ 57:267–273
Vogel EM, DWJ J, Gallagher PK (1977) Preparation, structure, and selected catalytic properties of the system LaMn1-xCuxO3-y. J Am Ceram Soc 60:31–33
Wu Z, Jin R, Wang H, Liu Y (2009) Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal Commun 10:935–939
Xu W, He H, Yu Y (2009) Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J Phys Chem C 113:4426–4432
Xu C, Sun W, Cao L, Li T, Cai X, Yang J (2017) Highly efficient Pd-doped aluminate spinel catalysts with different divalent cations for the selective catalytic reduction of NO with H2 at low temperature. Chem Eng J 308:980–987
Yao W, Wang R, Yang X (2009) LaCo1−xPdxO3 perovskite-type oxides: synthesis, characterization and simultaneous removal of NOx and diesel soot. Catal Lett 130:613–621
Zhang R, Alamdari H, Kaliaguine S (2006a) Fe-based perovskites substituted by copper and palladium for NO+CO reaction. J Catal 242:241–253
Zhang R, Villanueva A, Alamdari H, Kaliaguine S (2006b) SCR of NO by propene over nanoscale LaMn1−xCuxO3 perovskites. Appl Catal A Gen 307:85–97
Zhang R, Alamdari H, Kaliaguine S (2008a) SO2 poisoning of LaFe0.8Cu0.2O3 perovskite prepared by reactive grinding during NO reduction by C3H6. Appl Catal A Gen 340:140–151
Zhang Z, Jia Z, Wang Y, Wang F, Yang R (2008b) Microstructure and characterization of La2O3 and CeO2 doped diamond-like carbon nanofilms. Surf Coat Technol 202:5947–5952
Zhang Q, Lv L, Zhu J, Wang X, Wang J, Shen M (2013) The effect of CO on NO reduction over Pt/Pd-based NSR catalysts at low temperature. Catalysis Science & Technology 3:1069
Zhang R, Yang W, Luo N, Li P, Lei Z, Chen B (2014) Low-temperature NH3-SCR of NO by lanthanum manganite perovskites: effect of A-/B-site substitution and TiO2/CeO2 support. Appl Catal B Environ 146:94–104
Zhu Z, Niu H, Liu Z, Liu S (2000) Decomposition and reactivity of NH4HSO4 on V2O5/AC catalysts used for NO reduction with ammonia. J Catal 195:268–278
Zhu Y, Sun Y, Niu X, Yuan F, Fu H (2010) Preparation of La-Mn-O perovskite catalyst by microwave irradiation method and its application to methane combustion. Catal Lett 135:152–158
Zhua Y, Tana R, Feng J, Ji S, Cao L (2001) The reaction and poisoning mechanism of SO2 and perovskite LaCoO3 film model catalysts. Appl Catal A Gen 209:71–77
Acknowledgments
The authors thank Xuanxuan Cai and Baosheng Tu for their help.
Funding
This research was based on the work supported by the National Natural Science Foundation of China (21277045).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Li, T., Sun, W., Zhou, Z. et al. Di-metal-doped sulfur resisting perovskite catalysts for highly efficient H2-SCR of NO. Environ Sci Pollut Res 25, 25504–25514 (2018). https://doi.org/10.1007/s11356-018-2608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2608-8