Abstract
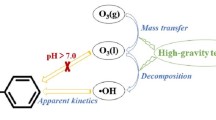
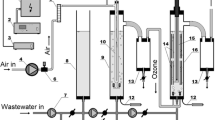
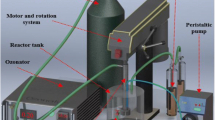
The rotating packed bed (RPB) as a continuous flow reactor performs very well in degradation of nitrobenzene wastewater. In this study, acidic nitrobenzene wastewater was degraded using ozone (O3) combined with hydrogen peroxide and titanium ions (Ti(IV)/H2O2/O3) or using only H2O2/O3 in a RPB. The degradation efficiency of nitrobenzene by Ti(IV)/H2O2/O3 is roughly 16.84% higher than that by H2O2/O3, and it reaches as high as 94.64% in 30 min at a H2O2/O3 molar ratio of 0.48. It is also found that the degradation efficiency of nitrobenzene is significantly affected by the high gravity factor, H2O2/O3 molar ratio, and Ti(IV) concentration, and it reaches a maximum at a high gravity factor of 40, a Ti(IV) concentration of 0.50 mmol/L, a pH of 4.0, a H2O2/O3 molar ratio of 0.48, a liquid flow rate of 120 L/h, and an initial nitrobenzene concentration of 1.22 mmol/L. Both direct ozonation and indirect ozonation are involved in the reaction of O3 with organic pollutants. The indirect ozonation due to the addition of different amounts of tert-butanol (·OH scavenger) in the system accounts for 84.31% of the degradation efficiency of nitrobenzene, indicating that the nitrobenzene is dominantly oxidized by ·OH generated in the RPB-Ti(IV)/H2O2/O3 process. Furthermore, the possible oxidative degradation mechanisms are also proposed to better understand the role of RPB in the removal of pollutants.

ᅟ











Similar content being viewed by others
References
Arslan I, Balcioglu IA, Tuhkanen T (2000) Advanced treatment of dyehouse effluents by Fe(II) and Mn(II)-catalyzed ozonation and the H2O2/O3 process. Water Sci Technol 42:13–18
Bircher KG, Bolton JR (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC technical report). Pure Appl Chem 73:627–637
Chen YH, Chang CY, Su WL, Chiu CY, Yu YH, Chiang PC, Chang CF, Shie JL, Chiou CS, Chiang SI (2005) Ozonation of CI reactive black 5 using rotating packed bed and stirred tank reactor. J Chem Technol Biotechnol 80:68–75
Chidambara Raj CB, Quen HL (2005) Advanced oxidation processes for wastewater treatment: optimization of UV/H2O2 process through a statistical technique. Chem Eng Sci 60:5305–5311
Elshafei GMS, Yehia FZ, Dimitry OIH, Badawi AM, Eshaq G (2014) Ultrasonic assisted-Fenton-like degradation of nitrobenzene at neutral pH using nanosized oxides of Fe and Cu. Ultrason Sonochem 21:1358–1365
García Einschlag FS, Lopez J, Carlos L, Capparelli AL (2002) Evaluation of the efficiency of photodegradation of nitroaromatics applying the UV/H2O2 technique. Environ Sci Technol 36:3936–3944
Guo L, Jiao WZ, Liu YZ, Xu CC, Liu WL, Li J (2015) Treatment of nitrobenzene-containing wastewater using different combined processes with ozone. Chin J Energ Mater 5:702–708
Hoigné J, Bader H, Haag WR, Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water-III. Non-dissociating compounds and radicals. Water Res 19:993–1004
Jiao WZ, Liu YZ, Qi GS (2010) Gas pressure drop and mass transfer characteristics in a cross-flow rotating packed bed with porous plate packing. Ind Eng Chem Res 49:3732–3740
Jiao WZ, Luo S, He Z, Liu YZ (2016) Applications of high gravity technologies for wastewater treatment: a review. Chem Eng J 313:912–927
Keen OS, Love NG, Aga DS, Linden KG (2015) Biodegradability of iopromide products after UV/H2O2 advanced oxidation. Chemosphere 144:989–994
Kordkandi SA, Ashiri R (2015) Modeling and kinetics study of acid anthraquinone oxidation using ozone: energy consumption analysis. Clean Technol Environ 17:2431–2439
Kordkandi SA, Forouzesh M (2014) Application of full factorial design for methylene blue dye removal using heat-activated persulfate oxidation. J Taiwan Inst Chem Eng 45:2597–2604
Kordkandi SA, Motlagh AM (2017) Optimization of peroxone reaction rate using metaheuristic approach in the dearomatization and discoloration process. Environ Prog Sustain Energy 37
Latifoglu A, Gurol MD (2003) The effect of humic acids on nitrobenzene oxidation by ozonation and O3/UV processes. Water Res 37:1879–1889
Li YM, Li XH, Wang Y, Chen YY, Ji JB, Yu YL, Xu ZC (2014) Distillation in a counterflow concentric-ring rotating bed. Ind Eng Chem Res 53:4821–4837
Lin SH, Wang CH (2003) Industrial wastewater treatment in a new gas-induced ozone reactor. J Hazard Mater 98:295–309
Lin CC, Chen BC, Chen YS, Hsu SK (2008) Feasibility of a cross-flow rotating packed bed in removing carbon dioxide from gaseous streams. Sep Purif Technol 62:507–512
Lin WW, Li PP, Zhang H, Shi R, Tong SP (2010) Degradation of acetic acid by Ti(IV)-catalyzed H2O2/O3. Chin J Chem. Ind Eng 61:1790–1795
Liu Y, He X, Fu YS, Dionysiou DD (2016) Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem Eng J 284:1317–1327
Luo Y, Chu GW, Zou HK, Xiang Y, Shao L, Chen JF (2012) Characteristics of a two-stage counter-current rotating packed bed for continuous distillation. Chem Eng Process 52:55–62
Ma J, Graham NJD (2000) Degradation of atrazine by manganese-catalysed ozonation-influence of radical scavengers. Water Res 34:3822–3828
Mandal BP, Biswas AK, Bandyopadhyay SS (2004) Selective absorption of H2S from gas streams containing H2S and CO2, into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Sep Purif Technol 35:191–202
Martins RC, Quinta-Ferreira RM (2011) Remediation of phenolic wastewaters by advanced oxidation processes (AOPs) at ambient conditions: comparative studies. Chem Eng Sci 66:3243–3250
Mondal A, Pramanik A, Bhowal A, Datta S (2012) Distillation studies in rotating packed bed with split packing. Chem Eng Res Des 90:453–457
Pera-Titus M, GarciA-Molina V, Barios MA, Giménez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B Environ 47:219–256
Rosenfeldt EJ, Linden KG, Canonica S, Gunten UV (2006) Comparison of the efficiency of ·OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res 40:3695–3704
Stefan MI, John M, James RB (2015) Degradation pathways during the treatment of methyl-tert-butyl ether by the UV/H2O2 process. Environ Sci Technol 34:650–658
Tong SP, Li WW, Zhao SQ, Ma CA (2011) Titanium(IV)-improved H2O2/O3 process for acetic acid degradation under acid conditions. Ozone Sci Eng 33:441–448
Wang H, Liu YZ, Meng XL, Liu HX, Jiao WZ (2008) Mass transfer model of ozone oxidation treatment of trinitrotoluene alkaline wastewater in a rotating packed bed. Chem Eng 12:6–9 (in chinese)
Xie J, Qu CT, Wang XQ (2005) Researches on degradation of nitrobenzene in aqueous solution using ultrasonic technology. Chem Ind Times 11:3–5 (in chinese)
Yang Y, Ma J, Qin Q, Zhai X (2007) Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J Mol Catal A Chem 267:41–48
Yang B, Zuo J, Li P, Wang K, Yu X, Zhang M (2016) Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem Eng J 287:30–37
Yao Y, Wang L, Sun L, Zhu S, Huang Z, Mao Y, Lu WY, Chen WX (2013) Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem Eng Sci 101:424–431
Yi F, Zou HK, Chu GW, Shao L, Chen JF (2009) Modeling and experimental studies on absorption of CO2 by benfield solution in rotating packed bed. Chem Eng J 145:377–384
Yuan MH, Chen YH, Tsai JY, Chang CY (2016) Ammonia removal from ammonia-rich wastewater by air stripping using a rotating packed bed. Process Saf Environ Prot 102:777–785
Zeng ZQ, Zou HK, Li X, Sun BC, Chen JF, Shao L (2012) Ozonation of phenol with O3/Fe(II) in acidic environment in a rotating packed bed. Ind Eng Chem Res 51:10509–10516
Zeng ZQ, Zou HK, Li X, Arowo M, Sun BC, Chen JF, Chu GW, Shao L (2013) Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed. Chem Eng J 229:404–401
Zhang YL, Zhang K, Dai CM, Zhou XF (2014a) Performance and mechanism of pyrite for nitrobenzene removal in aqueous solution. Chem Eng Sci 111:135–141
Zhang YL, Zhang K, Dai CM, Zhou XF, Si H (2014b) An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem Eng J 244:438–445
Funding
This work was supported by the Natural Science Foundations of China (U1610106) and Shanxi excellent talent science and technology innovation project (201705D211011), Specialized Research Fund for Sanjin Scholars Program of Shanxi Province (201707), and North University of China Fund for Distinguished Young Scholars (201701).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Highlights
• Ti(IV)/H2O2/O3 process coupled with RPB was developed to degrade acidic nitrobenzene.
• The degradation efficiency reached 94.64% with the nitrobenzene concentration of 1.22 mmol/L in 30 min.
• Direct reaction and indirect reaction coexisting in the oxidation system were confirmed.
• Indirect ozonation accounted for 84.31% in the whole oxidation process.
• The possible degradation pathways were proposed.
Rights and permissions
About this article
Cite this article
Yang, P., Luo, S., Liu, Y. et al. Degradation of nitrobenzene wastewater in an acidic environment by Ti(IV)/H2O2/O3 in a rotating packed bed. Environ Sci Pollut Res 25, 25060–25070 (2018). https://doi.org/10.1007/s11356-018-2551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2551-8