Abstract
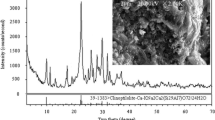
Spent magnesia (MgO)-carbon refractory bricks were repurposed as a permeable reactive barrier reactive media to treat a nickel (5 mg l−1)- and cobalt (0.3 mg l−1)-contaminated groundwater. MgO has been used for decades as a heavy metal precipitating agent as it hydrates and buffers the pH in a range of 8.5–10 associated with the minimum solubility of various divalent metals. The contaminated groundwater site’s conditions are typical of contaminated neutral drainage with a pH of 6 as well as high concentrations of iron (220 mg l−1) and sulphates (2500 mg l−1). Using synthetic contaminated water, batch and small-scale column tests were performed to determine the treatment efficiency and longevity. The increase and stabilization of the pH at 10 observed during the tests are associated with the hydration and dissolution of the MgO and promoted the removal not only of a significant proportion of the contaminants but also of iron. During the column test, this accumulation of precipitates over time clogged and passivated the MgO resulting in a loss of chemical performance (pH lowering, metal breakthrough) after 210 pore volumes of filtration. Precipitation also affected the hydraulic conductivity values which dropped from 2.3·10−3 to 4.2·10−4 m s−1 at the end of test. Saturation indices and XRD analyses suggest the precipitates formed are likely composed of goethite as well as iron, cobalt and nickel hydroxides. Recycled MgO-C refractory bricks were demonstrated to be an efficient reactive material for the removal of Co and Ni, but careful considerations should be taken of the potential clogging and passivation phenomena given particular physicochemical conditions.









Similar content being viewed by others
References
Bildstein O (1998) Modélisation géochimique des interactions eau-gaz-roche Application a la diagenèse minérale dans les réservoirs géologiques. Université de Strasbourg 1 (in French)
Blowes DW, Ptacek CJ, Benner SG, McRae CWT, Bennett TA, Puls RW (2000) Treatment of inorganic contaminants using permeable reactive barriers. J Contam Hydrol 45:123–137
Calugaru IL, Neculita CM, Genty T, Bussière B, Potvin R (2016) Performance of thermally activated dolomite for the treatment of Ni and Zn in contaminated neutral drainage. J Hazard Mater 310:48–55
Calugaru IL, Neculita CM, Genty T, Zagury GJ (2018) Metals and metalloids treatment in contaminated neutral effluents using modified materials. J Environ Manag 212:142–159
Caraballo MA, Rötting TS, Silva V (2010) Implementation of an MgO-based metal removal step in the passive treatment system of Shilbottle, UK: Column experiments. J Hazard Mater 181:923–930
Chimenos J, Fernandez A, Villalba G, Segarra M, Urruticoechea A, Artaza B, Espiell F (2003) Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res 37:1601–1607
Cortina J-L, Lagreca I, De Pablo J, Cama J, Ayora C (2003) Passive in situ remediation of metal-polluted water with caustic magnesia: evidence from column experiments. Environ Sci Technol 37:1971–1977
Courcelles B, Modaressi-Farahmand-Razavi A, Gouvenot D, Esnault-Filet A (2011) Influence of precipitates on hydraulic performance of permeable reactive barrier filters. Int J Geomech 11:142–151
Davies PJ, Bubela B (1973) The transformation of nesquehonite into hydromagnesite. Chem Geol 12:289–300
de Repentigny C, Courcelles B (2014) A simplified model to predict clogging of reactive barriers. Environ Geotechn 3:166–177
del Valle-Zermeño R, Giró-Paloma J, Formosa J, Chimenos J (2015) Low-grade magnesium oxide by-products for environmental solutions: characterization and geochemical performance. J Geochem Explor 152:134–144
Faghihi-Sani M-A, Yamaguchi A (2002) Oxidation kinetics of MgO–C refractory bricks. Ceram Int 28:835–839
Gibert O, de Pablo J, Luis Cortina J, Ayora C (2003) Evaluation of municipal compost/limestone/iron mixtures as filling material for permeable reactive barriers for in-situ acid mine drainage treatment. J Chem Technol Biotechnol 78:489–496
Gustafsson J (2011) Visual MINTEQ ver. 3.0 KTH Department of Land and Water Resources Engineering, Stockholm, Sweden Based on de Allison JD, Brown DS, Novo-Gradac KJ, MINTEQA2 ver 4, 1991
Hänchen M, Prigiobbe V, Baciocchi R, Mazzotti M (2008) Precipitation in the Mg-carbonate system—effects of temperature and CO2 pressure. Chem Eng Sci 63:1012–1028
ITRC (2011) Permeable reactive barrier: technology update. The Interstate Technology & Regulatory Council, PRB: Technology Update Team, Washington, D.C.
Jin F, Al-Tabbaa A (2014) Characterisation of different commercial reactive magnesia. Adv Cem Res 26:101–113
Kojima Y, Sadotomo A, Yasue T, Arai Y (1992) Control of crystal shape and modification of calcium carbonate prepared by precipitation from calcium hydrogencarbonate solution. J Ceram Soc Jpn 100:1145–1153
Macías F, Caraballo MA, Rötting TS, Pérez-López R, Nieto JM, Ayora C (2012) From highly polluted Zn-rich acid mine drainage to non-metallic waters: implementation of a multi-step alkaline passive treatment system to remediate metal pollution. Sci Total Environ 433:323–330
MOE (2011) Soil, ground water and sediment standards for use under part XV.1 of the Environmental Protection Act. Table 9—generic site condition standards for use within 30 m of a water body in a non-potable ground water condition. Ontario Ministry of the Environment
Navarro A, Chimenos JM, Muntaner D, Fernandez AI (2006) Permeable reactive barriers for the removal of heavy metals: lab-scale experiments with low-grade magnesium oxide. Ground Water Monit Remediat 26:142–152
Obiri-Nyarko F, Grajales-Mesa SJ, Malina G (2014) An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 111:243–259
Oustadakis P, Agatzini-Leonardou S, Tsakiridis PE (2006) Nickel and cobalt precipitation from sulphate leach liquor using MgO pulp as neutralizing agent. Miner Eng 19:1204–1211
Rocha SD, Mansur MB, Ciminelli VS (2004) Kinetics and mechanistic analysis of caustic magnesia hydration. J Chem Technol Biotechnol 79:816–821
Rötting TS, Ayora C, Carrera J (2008) Improved passive treatment of high Zn and Mn concentrations using caustic magnesia (MgO): particle size effects. Environ Sci Technol 42:9370–9377
Rötting TS, Cama J, Ayora C, Cortina J-L, De Pablo J (2006) Use of caustic magnesia to remove cadmium, nickel, and cobalt from water in passive treatment systems: column experiments. Environ Sci Technol 40:6438–6443
Schiller JE, Tallman DN, Khalafalla SE (1984) Mineral processing water treatment using magnesium oxide. Environ Prog 3:136–141
Terringo J III (1987) Magnesium hydroxide reduces sludge/improves filtering. Pollut Eng 19:78–83
USEPA (2008) Incorporating sustainable environmental practices into remediation of contaminated sites. U.S. Environmental Protection Agency. Office of Solid Waste and Emergency Response
Wray JL, Daniels F (1957) Precipitation of calcite and aragonite. J Am Chem Soc 79:2031–2034
Zagury GJ, Colombano SM, Narasiah KS, Ballivy G (1997) Neutralization of acid mine tailings by addition of alkaline sludges from pulp and paper industry. Environ Technol 18:959–973
Zagury GJ, Kulnieks VI, Neculita CM (2006) Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 64:944–954
Acknowledgements
The authors would like to thank the Quebec Fonds de Recherche Nature et Technologies, the Natural Sciences and Engineering Research Council of Canada and BluMetric Environmental Inc. for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
de Repentigny, C., Courcelles, B. & Zagury, G.J. Spent MgO-carbon refractory bricks as a material for permeable reactive barriers to treat a nickel- and cobalt-contaminated groundwater. Environ Sci Pollut Res 25, 23205–23214 (2018). https://doi.org/10.1007/s11356-018-2414-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2414-3