Abstract
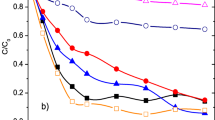
In this study, the effect of Fe3+, Fe2+, and Mn2+ dose, solution pH, reaction temperature, background water matrix (i.e., inorganic anions, cations, and natural organic matters (NOM)), and the kinetics and mechanism for the reaction system of Fe(VI)/Fe3+, Fe(VI)/Fe2+, and Fe(VI)/Mn2+ were investigated systematically. Traces of Fe3+, Fe2+, and Mn2+ promoted the DCF removal by Fe(VI) significantly. The pseudo-first-order rate constant (kobs) of DCF increased with decreasing pH (9–6) and increasing temperature (10–30 °C) due to the gradually reduced stability and enhanced reactivity of Fe(VI). Cu2+ and Zn2+ ions evidently improved the DCF removal, while CO32− restrained it. Besides, SO42−, Cl−, NO3−, Mg2+, and Ca2+ almost had no influence on the degradation of DCF by Fe(VI)/Fe3+, Fe(VI)/Fe2+, and Fe(VI)/Mn2+ within the tested concentration. The addition of 5 or 20 mg L−1 NOM decreased the removal efficiency of DCF. Moreover, Fe2O3 and Fe(OH)3, the by-products of Fe(VI), slightly inhibited the DCF removal, while α-FeOOH, another by-product of Fe(VI), showed no influence at pH 7. In addition, MnO2 and MnO4−, the by-products of Mn2+, enhanced the DCF degradation due to catalysis and superposition of oxidation capacity, respectively. This study indicates that Fe3+ and Fe2+ promoted the DCF removal mainly via the self-catalysis for Fe(VI), and meanwhile, the catalysis of Mn2+ and the effect of its by-products (i.e., MnO2 and MnO4−) contributed synchronously for DCF degradation.

ᅟ









Similar content being viewed by others
References
Aguinaco A, Beltrán FJ, García-Araya JF, Oropesa A (2012) Photocatalytic ozonation to remove the pharmaceutical diclofenac from water: influence of variables. Chem Eng J 189-190:275–282
Anquandah GA, Sharma VK, Knight DA, Batchu SR, Gardinali PR (2011) Oxidation of trimethoprim by ferrate(VI): kinetics, products, and antibacterial activity. Environ Sci Technol 45:10575–10581
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in aqueous solution). J Phys Chem Ref Data 17:513–886
Carr JD, Kelter PB, Tabatabai A, Splichal D, Erickson J, McLaughlin CW (1985) Properties of ferrate (VI) in aqueous solution: an alternate oxidant in wastewater treatment. In: Jolley RL (ed) Proceedings of Conference on Water Chlorination Chem. Environment Impact Health Eff. Lewis, Chelsew, pp 1285–1298
Cheng H, Song D, Liu H, Qu J (2015) Permanganate oxidation of diclofenac: the pH-dependent reaction kinetics and a ring-opening mechanism. Chemosphere 136:297–304
Feng M, Cizmas L, Wang Z, Sharma VK (2017a) Activation of ferrate(VI) by ammonia in oxidation of flumequine: kinetics, transformation products, and antibacterial activity assessment. Chem Eng J 323:584–591
Feng M, Cizmas L, Wang Z, Sharma VK (2017b) Synergistic effect of aqueous removal of fluoroquinolones by a combined use of peroxymonosulfate and ferrate(VI). Chemosphere 177:144–148
Feng M, Sharma VK (2018) Enhanced oxidation of antibiotics by ferrate(VI)-sulfur(IV) system: elucidating multi-oxidant mechanism. Chem Eng J 341:137–145
Filip J, Yngard RA, Siskova K, Marusak Z, Ettler V, Sajdl P, Sharma VK, Zboril R (2011) Mechanisms and efficiency of the simultaneous removal of metals and cyanides by using ferrate(VI): crucial roles of nanocrystalline iron(III) oxyhydroxides and metal carbonates. Chemistry 17:10097–10105
Gan W, Sharma VK, Zhang X, Yang L, Yang X (2015) Investigation of disinfection byproducts formation in ferrate(VI) pre-oxidation of NOM and its model compounds followed by chlorination. J Hazard Mater 292:197–204
García-Araya JF, Beltrán FJ, Aguinaco A (2010) Diclofenac removal from water by ozone and photolytic TiO2 catalysed processes. J Chem Technol Biotechnol 85:798–804
Goodwill JE, Mai X, Jiang Y, Reckhow DA, Tobiason JE (2016) Oxidation of manganese(II) with ferrate: stoichiometry, kinetics, products and impact of organic carbon. Chemosphere 159:457–464
Graham N, Jiang CC, Li XZ, Jiang JQ, Ma J (2004) The influence of pH on the degradation of phenol and chlorophenols by potassium ferrate. Chemosphere 56:949–956
Gupta H, Gupta B (2015) Photocatalytic degradation of polycyclic aromatic hydrocarbon benzo[a]pyrene by iron oxides and identification of degradation products. Chemosphere 138:924–931
Guyer GT, Ince NH (2011) Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason Sonochem 18:114–119
Han Q, Wang H, Dong W, Liu T, Yin Y (2014) WITHDRAWN: suppression of bromate formation in ozonation process by using ferrate(VI): batch study. Chem Eng J 236:110–120
Ho C-M, Lau T-C (2000) Lewis acid activated oxidation of alkanes by barium ferrate. New J Chem 24:587–590
Horst C, Sharma VK, Baum JC, Sohn M (2013) Organic matter source discrimination by humic acid characterization: synchronous scan fluorescence spectroscopy and ferrate(VI). Chemosphere 90:2013–2019
Hrostowski HJ, Scott AB (1950) The magnetic susceptibility of potassium ferrate. J Chem Phys 18:105–107
Huang J, Wang Y, Liu G, Chen P, Wang F, Ma J, Li F, Liu H, Lv W (2017) Oxidation of indometacin by ferrate (VI): kinetics, degradation pathways and toxicity assessment. Environ Sci Pollut Res Int 24:10786–10795
Huguet M, Deborde M, Papot S, Gallard H (2013) Oxidative decarboxylation of diclofenac by manganese oxide bed filter. Water Res 47:5400–5408
Jain A, Sharma VK, Mbuya OS (2009) Removal of arsenite by Fe(VI), Fe(VI)/Fe(III), and Fe(VI)/Al(III) salts: effect of pH and anions. J Hazard Mater 169:339–344
Jiang J-Q, Stanford C, Alsheyab M (2009) The online generation and application of ferrate(VI) for sewage treatment—a pilot scale trial. Sep Purif Technol 68:227–231
Jiang Y, Goodwill JE, Tobiason JE, Reckhow DA (2015) Effect of different solutes, natural organic matter, and particulate Fe(III) on ferrate(VI) decomposition in aqueous solutions. Environ Sci Technol 49:2841–2848
Johnson MD, Lorenz BB (2015) Antimony remediation using ferrate(VI). Sep Sci Technol 50:1611–1615
Kim C, Panditi VR, Gardinali PR, Varma RS, Kim H, Sharma VK (2015) Ferrate promoted oxidative cleavage of sulfonamides: kinetics and product formation under acidic conditions. Chem Eng J 279:307–316
Kralchevska RP, Prucek R, Kolarik J, Tucek J, Machala L, Filip J, Sharma VK, Zboril R (2016) Remarkable efficiency of phosphate removal: ferrate(VI)-induced in situ sorption on core-shell nanoparticles. Water Res 103:83–91
Lan B, Wang Y, Wang X, Zhou X, Kang Y, Li L (2016) Aqueous arsenic (As) and antimony (Sb) removal by potassium ferrate. Chem Eng J 292:389–397
Lee Y, Kissner R, von Gunten U (2014) Reaction of ferrate(VI) with ABTS and self-decay of ferrate(VI): kinetics and mechanisms. Environ Sci Technol 48:5154–5162
Luo Z, Li X, Zhai J (2016) Kinetic investigations of quinoline oxidation by ferrate(VI). Environ Technol 37:1249–1256
Manoli K, Nakhla G, Feng M, Sharma VK, Ray AK (2017a) Silica gel-enhanced oxidation of caffeine by ferrate(VI). Chem Eng J 330:987–994
Manoli K, Nakhla G, Ray AK, Sharma VK (2017b) Enhanced oxidative transformation of organic contaminants by activation of ferrate(VI): possible involvement of Fe V /Fe IV species. Chem Eng J 307:513–517
Manoli K, Nakhla G, Ray AK, Sharma VK (2017c) Oxidation of caffeine by acid-activated ferrate(VI): effect of ions and natural organic matter. AICHE J 63:4998–5006
Michael I, Achilleos A, Lambropoulou D, Torrens VO, Pérez S, Petrović M, Barceló D, Fatta-Kassinos D (2014) Proposed transformation pathway and evolution profile of diclofenac and ibuprofen transformation products during (sono)photocatalysis. Appl Catal B Environ 147:1015–1027
Nie E, Yang M, Wang D, Yang X, Luo X, Zheng Z (2014) Degradation of diclofenac by ultrasonic irradiation: kinetic studies and degradation pathways. Chemosphere 113:165–170
Noorhasan NN, Sharma VK (2008) Kinetics of the reaction of aqueous iron(vi) (FeVIO42-) with ethylenediaminetetraacetic acid. Dalton Trans 1883–1887. https://doi.org/10.1039/b715154c
Noorhasan N, Patel B, Sharma VK (2010) Ferrate(VI) oxidation of glycine and glycylglycine: kinetics and products. Water Res 44(3):927–935
Pochard I, Denoyel R, Couchot P, Foissy A (2002) Adsorption of barium and calcium chloride onto negatively charged α-Fe2O3 particles. J Colloid Interface Sci 255:27–35
Prucek R, Tucek J, Kolarik J, Huskova I, Filip J, Varma RS, Sharma VK, Zboril R (2015) Ferrate(VI)-prompted removal of metals in aqueous media: mechanistic delineation of enhanced efficiency via metal entrenchment in magnetic oxides. Environ Sci Technol 49:2319–2327
Rizzo L, Meric S, Kassinos D, Guida M, Russo F, Belgiorno V (2009) Degradation of diclofenac by TiO(2) photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res 43:979–988
Rush JD, Zhao Z, Bielski BHJ (1996) Reaction of ferrate(VI)/ferrate(V) with hydrogen peroxide and superoxide anion-a stopped-flow and premix pulse radiolysis study. Free Rad Res 24:187–198
Sharma VK, O’Connor DB (2000) Ferrate(V) oxidation of thiourea: a premix pulse radiolysis study. Inorg Chim Acta 311:40–44
Sharma VK, Burnett CR, Millero FJ (2001) Dissociation constants of the monoprotic ferrate(VI) ion in NaCl media. Phys Chem Chem Phys 3:2059–2062
Sharma VK (2011) Oxidation of inorganic contaminants by ferrates (VI, V, and IV)—kinetics and mechanisms: a review. J Environ Manag 92:1051–1073
Sharma VK (2013) Ferrate(VI) and ferrate(V) oxidation of organic compounds: kinetics and mechanism. Coord Chem Rev 257:495–510
Sharma VK, Chen L, Zboril R (2015a) Review on high valent FeVI (ferrate): a sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain Chem Eng 4:18–34
Sharma VK, Zboril R, Varma RS (2015b) Ferrates: greener oxidants with multimodal action in water treatment technologies. Acc Chem Res 48:182–191
Shu Z, Bolton JR, Belosevic M, El Din MG (2013) Photodegradation of emerging micropollutants using the medium-pressure UV/H2O2 advanced oxidation process. Water Res 47:2881–2889
Snyder SA, Adham S, Redding AM, Cannon FS, DeCarolis J, Oppenheimer J, Wert EC, Yoon Y (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181
Song Y, Deng Y, Jung C (2016) Mitigation and degradation of natural organic matters (NOMs) during ferrate(VI) application for drinking water treatment. Chemosphere 146:145–153
Soufan M, Deborde M, Legube B (2012) Aqueous chlorination of diclofenac: kinetic study and transformation products identification. Water Res 46:3377–3386
Wang H, Liu Y, Jiang JQ (2016) Reaction kinetics and oxidation product formation in the degradation of acetaminophen by ferrate (VI). Chemosphere 155:583–590
Wang Y, Liu H, Liu G, Xie Y, Gao S (2015) Oxidation of diclofenac by potassium ferrate (VI): reaction kinetics and toxicity evaluation. Sci Total Environ 506-507:252–258
Weast RC (1970-1971) Handbook of Chemistry and Physics, vol 51. CRC Press, Cleveland, p 111
Wood RH (1958) The heat, free energy, and entropy of the ferrate(VI) ion. J Amer Chem Soc 80:2038–2041
Xu GR, Zhang YP, Li GB (2009) Degradation of azo dye active brilliant red X-3B by composite ferrate solution. J Hazard Mater 161:1299–1305
Yang B, Ying GG (2013) Oxidation of benzophenone-3 during water treatment with ferrate(VI). Water Res 47:2458–2466
Yang Y-J, Liu E-H, Li L-M, Huang Z-Z, Shen H-J, Xiang X-X (2010) Nanostructured amorphous MnO2 prepared by reaction of KMnO4 with triethanolamine. J Alloys Compd 505:555–559
Yu W, Yang Y, Graham N (2016) Evaluation of ferrate as a coagulant aid/oxidant pretreatment for mitigating submerged ultrafiltration membrane fouling in drinking water treatment. Chem Eng J 298:234–242
Zhang P, Zhang G, Dong J, Fan M, Zeng G (2012) Bisphenol A oxidative removal by ferrate (Fe(VI)) under a weak acidic condition. Sep Purif Technol 84:46–51
Zhang T, Zhu H, Croue JP (2013) Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: efficiency, stability, and mechanism. Environ Sci Technol 47:2784–2791
Zhang Y, Geissen SU, Gal C (2008) Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73:1151–1161
Zhao J, Liu Y, Wang Q, Fu Y, Lu X, Bai X (2018) The self-catalysis of ferrate (VI) by its reactive byproducts or reductive substances for the degradation of diclofenac: kinetics, mechanism and transformation products. Sep Purif Technol 192:412–418
Zhou JH, Chen KB, Hong QK, Zeng FC, Wang HY (2017) Degradation of chloramphenicol by potassium ferrate (VI) oxidation: kinetics and products. Environ Sci Pollut Res Int 24:10166–10171
Funding
This work was supported by the Key R & D project of Science and Technology Department of Sichuan Province [No. 2017SZ0175], the Science and Technology Huimin Project of Chengdu [No. 2014-HM01-00278-SF], and the Joint Research Program about Sustainable Use of Water and Resources in the Upper Yangtze River [No. 2012DFG91520].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Vítor Pais Vilar
Highlights
• DCF removal using Fe(VI) was promoted by Cu2+ and Zn2+ but restrained by CO32−.
• Fe2O3 and Fe(OH)3 inhibited DCF removal, while α-FeOOH showed no influence at pH 7.
• MnO2 and MnO4− enhanced DCF oxidation using Fe(VI) via catalysis and superposition of oxidizability.
• Fe3+ and Fe2+ promoted DCF removal efficiency mainly through the self-catalysis of Fe(VI).
• The catalysis of Mn2+ and the effect of by-products contributed for DCF degradation greatly.
Rights and permissions
About this article
Cite this article
Zhao, J., Wang, Q., Fu, Y. et al. Kinetics and mechanism of diclofenac removal using ferrate(VI): roles of Fe3+, Fe2+, and Mn2+. Environ Sci Pollut Res 25, 22998–23008 (2018). https://doi.org/10.1007/s11356-018-2375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2375-6