Abstract
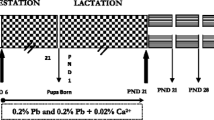
The present study evaluated the protective effects of Centella asiatica (CA) leaf extract on behavioral deficits and neurotoxicity in adult rat exposed to lead during perinatal period. Adult Wistar rats were exposed to 0.15% lead acetate (Pb) from gestation day 6 through drinking water and the pups were exposed lactationally to Pb till weaning. Significant perturbations in locomotor activity and exploratory behavior were observed in rats exposed to Pb during perinatal period. The levels of lipid peroxidation increased significantly with a reduction in levels of glutathione and activity levels of acetylcholinesterase and antioxidant enzymes in hippocampus, cerebrum, cerebellum, and medulla of brains excised from Pb-exposed rats. Oral supplementation of CA during postweaning period provided significant protection against Pb-induced behavioral impairments and neurotoxicity, without chelating tissue Pb levels. The possible neuroprotective efficacy of CA may be due to its antioxidant potential but not by lowering effects of brain Pb content.







Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:125–126
Akerboom TP, Sies H (1981) Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Meth Enzymol 77:373–382
Altmann L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G (1993) Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett 66:105–112
Antonio MT, Corpas I, Leret ML (1999) Neurochemical changes in new born rat’s brain after gestational cadmium and lead exposure. Toxicol Lett 104:1–9
Basha DC, Usha Rani M, Devi CB, Ram Kumar M, Reddy GR (2012) Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: reversal effect of calcium co-administration. Int J Dev Neurosci 30:343–350
Bradbury MW, Deane R (1993) Permeability of the blood-brain barrier to lead. Neurotoxicology 14:131–136
Bull RJ, McCauley PT, Taylor DH, Croten KM (1983) The effects of lead on the developing central nervous system of the rat. Neurotoxicology 4:1–17
Burdette LJ, Goldstein R (1986) Long-term behavioral and electrophysiological changes associated with lead exposure at different stages of brain development in the rat. Brain Res 394:101–110
Carlberg I, Mannervik B (1985) Glutathione reductase. Meth Enzymol 113:484–490
Chikezie PC, Chiedozie P, Ibegbulem O, Mbagwu FN (2015) Bioactive principles from medicinal plants. Res J Phytochem 9:88–115
Chopra RN, Nayer SL, Chopra IC, Asolkar LV, Kakkar KK (1994) Glossary of Indian medicinal plants. CSIR Publications, New Delhi
CPCSEA (2003) Committee for the Purpose of Control And Supervision of Experiments on Animals: guidelines for laboratory animal facility. Indian J Pharm 35:257–274
Crumpton T, Atkin DS, Zawia NH, Barone JRS (2001) Lead exposure to pheochromocytoma (PC12) cells alters neural differentiation and Sp1 DNA-binding. Neurotoxicology 22:49–62
Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav 38:723–729
Duggina P, Kalla CM, Varikasavu SR, Bukke S, Tartte V (2015) Protective effect of Centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: its possible mechanisms of action. J Physiol Biochem 71:435–454
Ellman GL, Courtney KD, Andres VRM, Featherstone RM (1961) A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Espejo EF, Mir D (1993) Structure of rat’s behavior in the hot plate test. Behav Brain Res 56:171–176
File SE, Kenny PJ, Cheeta S (2000) The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66:65–72
Fjerdingstad EJ, Danscher G, Fjerdingstad E (1974) Hippocampus: selective concentration of lead in the normal rat brain. Brain Research 80(2):350–354
Flohe L, Gulzer WA (1984) Glutathione peroxidase. Methods Enzymol 105:114–121
Flora SJS, Gupta R (2007) Beneficial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother Res 21:980–988
Hermes-Lima M, Pereira B, Bechara EJH (1991) Are free radicals involved in lead poisoning? Xenobiotica 21:1085–1090
Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH (2007) Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem 100:535–541
Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ (1996) Determination of biologically active constituents in Centella asiatica. J Chromatogr 742:127–130
James JT, Dubery IA (2009) Pentacyclictriterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 14:3922–3941
Jin Y, Yu F, Liao Y, Liu S, Liu M, Xu J, Yang J (2011) Therapeutic efficiency of succimer used with calcium and ascorbic acid in the treatment of mild lead poisoning. Environ Toxicol Pharmacol 31:137–142
Krigman M, Bouldin TW, Mushak P (1980) Lead. In: Spencer P, Schaumburg H (eds) Experimental and clinical neurotoxicology. Williams & Wilkins, Baltimore, pp 490–507
Krishnamurthy RG, Senut MC, Zemke D, Min J, Frenkel MB, Greenberg EJ, Yu SW, Ahn N, Goudreau J, Kassab M, Panickar KS, Majid A (2009) Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 87:2541–2550
Kumar MH, Gupta YK (2003) Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethanopharmacol 79:253–260
Kumar A, Dogra S, Prakash A (2009) Neuro protective effects of Centella asiatica against intra cerebro-ventricular colchicine induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2009:1–8
Kuroda M, Mimaki Y, Harada H, Sakagami H, Sashida Y (2001) Five new triterpene glycosides from Centella asiatica. Nat Med 55:134–138
Leret ML, Antonia JSM, Antonia MT (2003) Perinatal exposure to lead and cadmium affects anxiety-like behavior. Behav Toxicol 186:125–130
Lidsky TI, Schineder JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Lowry LH, Rosebrough NI, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Ma T, Chen HH, Ho IK (1999) Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. Toxicol Lett 105:111–121
Marques NF, Stefanello ST, Froeder AL, Busanello A, Boligon AA, Athayde ML, Soares PA, Fachinetto R (2015) Centella asiatica and its fractions reduces lipid peroxidation induced by quinolinic acid and sodium nitroprusside in rat brain regions. Neurochem Res 40:1197–1210
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23:489–495
Morris RGM (1984) Developments of water maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Mylroie AA, Umbles C, Kyle J (1984) Effects of dietary copper supplementation on erythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate. In: Hemphill DD (ed) Trace substances in environmental health, vol 18. University of Mussouri Press, Columbia, pp 497–504
Nichols DM, McLachlan DRXC (1990) Issues of lead toxicity. In: Yasumura S (ed) In vivo body composition studies. Plenum Press, New York, pp 237–246
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Orhan IE (2012) Centella asiatica (L.): from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Altern Med 2012:1–8
Petit TL, Alfano DP, Leboutillier JC (1983) Early lead exposure and the hippocampus: a review and recent advances. Neurotoxicology 4:79–94
Prasanthi RPJ, Devi CB, Basha RM, Reddy GR (2010) Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci 28:161–167
Ramana KD, Singhal SS, Reddy AB (2014) Therapeutic potential of natural pharmacological agents in the treatment of human diseases. BioMed Res Int 1–4, Article ID 573452
Rao KGM, Rao MS, Rao SG (2005) Centella asiatica (Linn) induced behavioural changes during growth spurt period in neonatal rats. Neuroanatomy 4:18–23
Reckziegel P, Dias VT, Benvegnu D, Boufleur N, Barcelos RCS, Segat HJ, Pase CS, Dos Santos CMM, Flore EMM, Burger ME (2011) Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett 203:74–81
Reddy GR, Basha MR, Devi CB, Suresh A, Baker JL, Shafeek A, Heinz J, Chetty S (2003) Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int J Dev Neurosci 21:347–352.
Reshma Anjum M, Reddy PS (2013) Effect of perinatal exposure to lead acetate on testicular lipid peroxidation adult rats. Int J Pharm Bio Sci 4:893–898
Rodrigues ALS, Rocha MA, Souza DA, Mello CF (1996) Effect of perinatal lead exposure on rat behaviour in open field and two way avoidance tasks. Pharmacol Toxicol 79:150–156
Ronis MJ, Badger TM, Robertson PK, Sheikh F (1996) Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol Appl Pharmacol 136:361–371
Ryzhavskii B, Lebedko OA, Belolyubskaya DS, Baranova SN (2008) Long-term consequences of prenatal exposure to lead on brain development in rats. Neurosci Behav Physiol 38:145–148
Sandhir R, Julka D, Gill KD (1994) Lipoperoxidative damage on lead treatment in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol 74:66–71
Sansar W, Gamrani H (2003) The pharmacological effect of Artemisia absinthium extract in protecting adult rats against lead neurotoxicity. J Neurol Sci 333:e598
Shih TM, Hanin I (1978) Effects of chronic lead exposure on levels of acetylcholine and choline and on acetylcholine turnover rate in rat brain areas in vivo. Psychopharmacology 58:263–269
Shinomol GK, Muralidhara K (2008) Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and it’s in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine 15:971–984
Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN (1999) In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 65:1–11
Sivaprasad R, Nagaraj M, Varalakshmi P (2002) Lipoic acid in combination with a chelator ameliorates lead-induced peroxidative damages in rat kidney. Arch Toxicol 76:437–441
Sivaprasad R, Nagaraj M, Varalakshmi P (2004) Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem 15:18–23
Sugunabai J, Jeyaraj M, Karpagam T (2015) Analysis of functional compounds and antioxidant activity of Centella asiatica. World J Pharm Pharmceu Sci 4:1982–1993
Sun L, Zhao ZY, Hu J, Zhou XL (2005) Potential association of lead exposure during early development of mice with alteration of hippocampus nitric oxide levels and learning memory. Biomed Environ Sci 18:375–378
Veerendra Kumar MH, Gupta YK (2003) Effect of Centella asiatica on cognition and oxidative stress in an intra-cerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol 30:336–342
Verina T, Rohde CA, Guilarte TR (2007) Environmental Pb2+ exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145:1037–1047
Wang XS, Dong Q, Zuo JP, Fang N (2003) Structure and potential immunological activity of a pectin from Centella asiatica (L.) Urban. Carbohydr Res 338:2393–2402
Wijeweera P, Arnason JHT, Koszycki D, Merali Z (2006) Evaluation of anxiolytic properties of gotukola (Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine 13:668–676
Williams K, Wilson MA, Bressler J (2000) Regulation and developmental expression of the divalent metal ion transporter in the rat brain. Cell Mol Biol 46:563–571
Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L, Gao J (2012a) Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharm Sin 33:578–587
Xu CL, Wang QZ, Sun LM, Li XM, Deng JM, Li LF, Zhang J, Xu R, Ma PS (2012b) Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of Parkinsonism via maintaining redox balance and upregulating the ratio of Bcl-2/Bax. Pharmacol Biochem Behav 100:413–418
Acknowledgements
The authors thank Dr. K.V.S. Sarma, Professor (Statistics), S.V. University, Tirupati, for providing assistance in the statistical analysis. KPR and CHS are grateful to the University Grants Commission, New Delhi, for financial support in the form of UGC-BSR (RFMS) fellowships.
Author information
Authors and Affiliations
Contributions
PSR conceived the idea, provided facilities, and supervised the work. KPR maintained rat colony and treatments and performed behavioral studies and assayed AChE activity. CHS prepared CA extract, analyzed the extract, and determined the Pb levels. KPR prepared rough draft of manuscript and all the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
All experimental investigations were done in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India (CPCSEA 2003). The protocol was approved by the Institutional Animal Ethics Committee (Regd. No. 438/01/a/CPCSEA/dt.17.07.2001) in its resolution no: 57/2012/(i)/a/CPCSEA/IAEC/SVU/ PSR-KPR dt.08-07-2012. The study was planned and organized as completely double blind.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Chintapanti, S., Pratap Reddy, K. & Sreenivasula Reddy, P. Behavioral and neurochemical consequences of perinatal exposure to lead in adult male Wistar rats: protective effect by Centella asiatica. Environ Sci Pollut Res 25, 13173–13185 (2018). https://doi.org/10.1007/s11356-018-1500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1500-x