Abstract
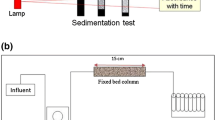
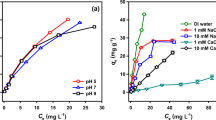
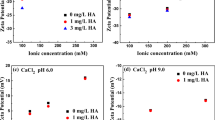
Colloidal particles have an ability to sorb heavy metals, metalloids, and organic compounds (e.g. biosurfactants) present in soil and groundwater. The pH and ionic strength changes may promote release of such particles causing potential contaminant transport. Therefore, it is very important to know how a colloid particle-mineral particle and colloid-mineral-biosurfactant system behaves in the natural environment. They can have negative impact on the environment and human health. This study highlighted the influence of biosurfactants produced by Pseudomonas aeruginosa on the transport of colloidal hematite (α-Fe2O3) through porous bed (materials collected from the Szklary and Zloty Stok solid waste heaps from Lower Silesia, Poland). Experiments were conducted using column set in two variants: colloid solution with porous bed and porous bed with adsorbed biosurfactants, in the ionic strengths of 5 × 10−4 and 5 × 10−3 M KCl. The zeta potential of mineral materials and colloidal hematite, before and after adsorption of biosurfactant, was determined. Obtained results showed that reduction in ionic strength facilitates colloidal hematite transport through the porous bed. The mobility of colloidal hematite was higher when the rhamnolipid adsorbed on the surface of mineral grain.









Similar content being viewed by others
References
Bordoloi KN, Konwar KB (2008) Microbial surfactant-enhanced oil recovery under laboratory conditions. Colloids Surf B 63:73–82
Chowdhury I, Hong Y, Honda JR, Walker LS (2011) Mechanisms of TiO2 nanoparticle transport in porous media: role of solution chemistry, nanoparticle concentration and flow rate. J Colloid Interface Sci 360:548–555
Dubois M, Gills AK, Hamilton KJ, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–352
Frey MJ, Schmitz P, Dufreche J, Gohr Pinheiro I (1999) Particle deposition in porous media: hydrodynamic and weak inertial effects. Transp Porous Media 37(1):25–54
Grolimund D, Borkovec M (2001) Release and transport of natural porous media: 1. Model Water Resour Res 37(3):559–570
Johnson RP, Sun N, Elimelech M (1996) Colloid transport in geochemically heterogeneous porous media: modeling and measurements. Environ Sci Technol 30:3284–3293
Markewitz K, Cabral RA, Panarotto TC, Lefebvre G (2004) Anaerobic biodegradation of an organic by-products leachate by interaction with different tailings. J Hazard Mater 110:93–104
Natarajan N, Suresh Kumar G (2010) Radionuclide and colloid co-transport in a coupled fracture-skin-matrix system. Colloids Surf A Physicochem Eng Asp 370:49–57
Sen R (2008) Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust Sci 34:714–724
Siebentritt M, Volovitch P, Ogle K, Lefevre G (2014) Adsorption and electroreduction of hematite particles on steel in strong alkaline media. Colloids Surf A Physicochem Eng Asp 440:197–201
Suchomel JB, Chen MB, Allen BM (1998a) Macroscale properties of porous media a network model of biofilm processes. Transp Porous Med 31:39–66
Suchomel JB, Chen MB, Allen BM (1998b) Network model of flow transport and biofilm effects in porous media. Transp Porous Med 30:1–23
Sugimoto T, Sakota K (1992) Preparation of monodisperse pseudocubic α-Fe2O3 particles from condensed ferric hydroxide. J Colloid Interface Sci 152:587–590
Sun Y, Petersen NJ, Bear J, Clement PT, Hooker SB (1999) Modeling microbial transport and biodegradation in a dual-porosity system. Transp Porous Med 35:49–65
Szymanska A, Sadowski Z (2010) Effect of biosurfactants on surface properties of hematite. Adsorption 16:233–239
Tripathy A (2010) Hydrodynamically and chemically induced in situ kaolin particle release from porous media an experimental study. Adv Powder Technol 21:564–572
Tufenkji N (2007) Modeling microbial transport in porous media: traditional approaches and recent development. Adv Water Resour 30:1455–1469
Wang S, Mulligan NC (2009) Rhamnolipid biosurfactant-enhanced soil flushing for removal of arsenic and heavy metals from mine tailings. Process Biochem 44:296–301
Acknowledgements
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of chemistry of Wroclaw University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Pawlowska, A., Sznajder, I. & Sadowski, Z. The colloid hematite particle migration through the unsaturated porous bed at the presence of biosurfactants. Environ Sci Pollut Res 24, 17912–17919 (2017). https://doi.org/10.1007/s11356-017-9435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9435-1