Abstract
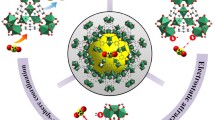
Two series of metal–organic frameworks (MOFs) with similar formula units but different central metal ions (M) or organic linkers (L), M-BDC (BDC = terephthalate, M = Zn, Zr, Cr, or Fe), or Zn-L (L = imidazolate-2-methyl, BDC, BDC-NH2), were prepared and employed as the receptors for adsorption lead ions. It was found that the Zn-BDC exhibited a much higher adsorption capacity than the other M-BDC series with various metal ions which have very closely low capacities at same conditions. Furthermore, the Zn-L (L = imidazolate-2-methyl, BDC, BDC-NH2) still have highly efficient adsorption capacity of lead ions, although the adsorption capacity varies with different ligand, as well as the adsorption rate and the equilibrium pH of the solution. This significant high adsorption over Zn-L, different from other M-BDC series with various metal ions (Zr, Cr, or Fe), can be explained by ion exchange between the central metal ions of Zn-L and lead ion in solution. Based on the analysis of FT-IR, X-ray diffraction pattern, the nitrogen adsorption isotherms, the zeta potentials, and the results, a plausible adsorption mechanism is proposed. When equivalent Zn-L were added to equal volume of aqueous solution with different concentration of lead ion, the content of zinc ion in the solution increases with the increase of the initial concentration of lead ions. The new findings could provide a potential way to fabricate new metal organic frameworks with high and selective capacities of the heavy metal ions.






Similar content being viewed by others
References
Ai L, Zhang C, Li L, Jiang J (2014) Iron terephthalate metal–organic framework: revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl Catal B-Environ 148-149:191–200
Arcibar-Orozco JA, Rangel-Mendez JR, Diaz-Flores PE (2015): Simultaneous adsorption of Pb(Ii)-Cd(Ii), Pb(Ii)-phenol, and Cd(Ii)-phenol by activated carbon cloth in aqueous solution. Water Air and Soil Pollution 226
Bakhtiari N, Azizian S (2015) Adsorption of copper ion from aqueous solution by nanoporous MOF-5: a kinetic and equilibrium study. J Mol Liq 206:114–118
Bortey-Sam N, Nakayama SM, Akoto O, Ikenaka Y, Fobil JN, Baidoo E, Mizukawa H, Ishizuka M (2015) Accumulation of heavy metals and metalloid in foodstuffs from agricultural soils around Tarkwa area in Ghana, and associated human health risks. Int J Environ Res Public Health 12:8811–8827
Chawla J, Kumar R, Kaur I (2015) Carbon nanotubes and graphenes as adsorbents for adsorption of lead ions from water: a review. J Water Supply Res Technol 64:641–659
Ding S, Dong Q, Hu J, Xiao W, Liu X, Liao L, Zhang N (2016) Enhanced selective adsorption of CO2 on nitrogen-doped porous carbon monoliths derived from IRMOF-3. Chem Commun (Camb) 52:9757–9760
Haider S, Park SY (2009) Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J Membr Sci 328:90–96
Han S, Lah MS (2015) Simple and efficient regeneration of MOF-5 and HKUST-1 via acid-base treatment. Cryst Growth Des 15:5568–5572
Haque E, Lee JE, Jang IT, Hwang YK, Chang JS, Jegal J, Jhung SH (2010) Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J Hazard Mater 181:535–542
Hasan Z, Jhung SH (2015) Removal of hazardous organics from water using metal-organic frameworks (MOFs): plausible mechanisms for selective adsorptions. J Hazard Mater 283:329–339
Ho Y-S, Chiang T-H, Hsueh Y-M (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40:119–124
Hu Y, Kazemian H, Rohani S, Huang Y, Song Y (2011) In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem Commun (Camb) 47:12694–12696
Huang L, Zhou Y, Guo XQ, Chen ZL (2015) Simultaneous removal of 2,4-dichlorophenol and Pb(II) from aqueous solution using organoclays: isotherm, kinetics and mechanism. J Ind Eng Chem 22:280–287
Jian MP, Liu B, Zhang GS, Liu RP, Zhang XW (2015) Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids and Surfaces a-Physicochemical and Engineering Aspects 465:67–76
Jiang J, Yaghi OM (2015) Bronsted acidity in metal-organic frameworks. Chem Rev 115:6966–6997
Jiang N, Deng Z, Liu S, Tang C, Wang G (2016) Synthesis of metal organic framework (MOF-5) with high selectivity for CO2/N2 separation in flue gas by maximum water concentration approach. Korean J Chem Eng:1–9
Jun JW, Tong M, Jung BK, Hasan Z, Zhong C, Jhung SH (2015) Effect of central metal ions of analogous metal-organic frameworks on adsorption of organoarsenic compounds from water: plausible mechanism of adsorption and water purification. Chemistry 21:347–354
Ke F, Qiu LG, Yuan YP, Peng FM, Jiang X, Xie AJ, Shen YH, Zhu JF (2011) Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J Hazard Mater 196:36–43
Khan NA, Hasan Z, Jhung SH (2013) Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): a review. J Hazard Mater 244-245:444–456
Khan NA, Jung BK, Hasan Z, Jhung SH (2015) Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks. J Hazard Mater 282:194–200
Lan YQ, Jiang HL, Li SL, Xu Q (2011) Mesoporous metal-organic frameworks with size-tunable cages: selective CO(2) uptake, encapsulation of ln(3)(+) cations for luminescence, and column-chromatographic dye separation. Adv Mater 23:5015–5020
Li C, Xiong ZH, Zhang JM, Wu CS (2015) The strengthening role of the amino group in metal-organic framework MIL-53 (Al) for methylene blue and malachite green dye adsorption. J Chem Eng Data 60:3414–3422
Lu R, Sheng GP, Liang Y, Li WH, Tong ZH, Chen W, Yu HQ (2013) Characterizing the interactions between polycyclic aromatic hydrocarbons and fulvic acids in water. Environ Sci Pollut R 20:2220–2225
Machida M, Fotoohi B, Amamo Y, Mercier L (2012) Cadmium(II) and lead(II) adsorption onto hetero-atom functional mesoporous silica and activated carbon. Appl Surf Sci 258:7389–7394
McGuire CV, Forgan RS (2015) The surface chemistry of metal-organic frameworks. Chem Commun (Camb) 51:5199–5217
Mueller U, Schubert M, Teich F, Puetter H, Schierle-Arndt K, Pastre J (2006) Metal-organic frameworks—prospective industrial applications. ChemInform 37:626–636
Nan D, Li H, Xiao F, Wang Q, Shan W, Li M, Zhou J, Bo W (2016): Partitioning MOF-5 into confined and hydrophobic compartments for carbon capture under humid conditions. Journal of the American Chemical Society 138
Naseem R, Tahir SS (2001) Removal of Pb(II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 35:3982–3986
Schröder M, Schröder M (2010): Functional metal organic frameworks: gas storage, separation and catalysis. Springer Berlin Heidelberg
Shen Y, Zhang Y, Zhang Q, Niu L, You T, Ivaska A (2005) Immobilization of ionic liquid with polyelectrolyte as carrier. Chem Commun (Camb):4193–4195
Wang XL, Fan HL, Tian Z, He EY, Li Y, Ju SG (2014a) Adsorptive removal of sulfur compounds using IRMOF-3 at ambient temperature. Appl Surf Sci 289:107–113
Wang Y, Jin S, Wang Q, Lu G, Jiang J, Zhu D (2013) Zeolitic imidazolate framework-8 as sorbent of micro-solid-phase extraction to determine estrogens in environmental water samples. J Chromatogr A 1291:27–32
Wang Y, Xie J, Wu YC, Hu XY (2014b) A magnetic metal-organic framework as a new sorbent for solid-phase extraction of copper(II), and its determination by electrothermal AAS. Microchim Acta 181:949–956
Wu CS, Xiong ZH, Li C, Zhang JM (2015) Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution. RSC Adv 5:82127–82137
Xian-Fa SU, Shi HL, Zhu GF, Gao YB, Jing F (2009) Study on the preparation of lead ion imprinted polymer and its specific adsorption performance. Metallurgical Analysis 29:16–20
Yang K, Xue F, Sun Q, Yue R, Lin D (2013) Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. Journal of Environmental Chemical Engineering 1:713–718
Yeom YH, Wen B, Sachtler WMH, Weitz E (2004) NOx reduction from diesel emissions over a nontransition metal zeolite catalyst: a mechanistic study using FTIR spectroscopy. Jphyschemb 108:5386–5404
Zhang HF, Liu DF, Yao Y, Zhang BQ, Lin YS (2015) Stability of ZIF-8 membranes and crystalline powders in water at room temperature. J Membr Sci 485:103–111
Zhao HH, Song HL, Chou LJ (2014) Facile synthesis of MOF-5 structure with large surface area in the presence of benzoyl peroxide by room temperature synthesis. Mater Chem Phys 143:1005–1011
Zhou HC, Long JR, Yaghi OM (2012) Introduction to metal-organic frameworks. Chem Rev 112:673–674
Zhou M, Wu YN, Qiao J, Zhang J, McDonald A, Li G, Li F (2013) The removal of bisphenol A from aqueous solutions by MIL-53 (Al) and mesostructured MIL-53 (Al). J Colloid Interface Sci 405:157–163
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 50878138).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Six kinds of MOFs (ZIF-8, MOF-5, IRMOF-3, UiO-66, MIL-101 (Cr), and MIL-53 (Fe)) are tested in the adsorption of Pb (II) in aqueous solution.
• Zinc-based MOFs, especially ZIF-8, show high selectivity in the adsorption of Pb (II).
• Kinetics and isotherms of Pb (II) adsorption over ZIF-8 are determined.
• The adsorption behavior of Pb (II) over MOFs is significantly affected by the pH of solution.
• The adsorption mechanism can be explained by ion exchange effect between Pb (II) and Zn (II) of the zinc-based MOFs.
Electronic supplementary material
.
ESM 1
(DOC 100 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Zhao, X., Zhang, J. et al. Selective adsorption of Pb (II) over the zinc-based MOFs in aqueous solution-kinetics, isotherms, and the ion exchange mechanism. Environ Sci Pollut Res 24, 14198–14206 (2017). https://doi.org/10.1007/s11356-017-9002-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9002-9