Abstract
Loss of suitable seminatural habitats and homogenization of crop types have led to the population decline of pollinating insects in farmland. As these insects support crop production, many practical efforts aim to sustain pollinator diversity which is especially challenging in intensively managed and homogeneous farmland. However, there are ongoing changes of the farmland toward its multifunctionality that includes, for example, wind farm development. Windmills are often built within crops; thus, we examined if the noncropped area around windmills can be valuable habitats for wild plants and pollinating insects: bees, butterflies, and flies. Species richness, abundances, and species diversity index of plants and pollinators around windmills were similar to those found in grassland patches (a typical habitat for these insects) and higher than in the adjacent crops. Pollinator diversity index and species richness at windmills increased with the distance to the nearest grassland patch and windmill. The population sizes of pollinating insects were also positively associated with plant diversity. Particular groups of pollinators showed specific habitat associations: bees occurred mostly at windmills, butterflies were highly associated with grasslands, while flies occurred in a similar number at windmill and on grasslands. Since windmills are frequently built within extensive homogeneous fields, thus, they introduce pollination services into the interior of cropped areas, contrary to field margins, road verges, or seminatural grasslands. Thus, although the development of wind farms has various negative environmental consequences, they can be alleviated by the increase of the local population size and diversity of wild plants and pollinating insects at windmills.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farmland is an important habitat for many taxa (Pimentel et al. 1992; Söderström et al. 2001; Scherr and McNeely 2008; Rosin et al. 2016a). However, the intensification of farming has led to habitat fragmentation, habitat loss, and pollution of farmland and, in a consequence, to collapse or diminishing of ecosystem services (Green et al. 2005; Stoate et al. 2009). One of the most threatened ecosystem service is pollination of plants, which plays a key role in the food production and sustaining wild plant species diversity (Potts et al. 2010; Sekercioglu et al. 2010; Baude et al. 2016). The estimated number of pollinator species reaches over 1200 for vertebrates and nearly 300,000 for invertebrates which are responsible for pollination of over 90% of flowering angiosperms and 95% of crop plants (Nabhan and Buchmann 1997). The most recognized groups of pollinators are bees, but there are also other taxa such as flies, butterflies, birds, or bats that may contribute to pollination substantially (Rader et al. 2016).
The literature highlights a number of factors responsible for the disappearance of pollinators, such as the use of pesticides, mechanical cultivation, parasites infection, unavailability of food plants, invasive plant species, or climate change (Kevan and Viana 2003; Moroń et al. 2009; Lenda et al. 2010), many of which are highly related to intensive agriculture practices. The most frequently mentioned factor is fragmentation and loss of habitats, mainly seminatural grasslands with evidences that rising distance to those habitats in homogeneous farmland causes disturbances in plant-pollinator interactions and reduces the production of seeds and fruits (Ricketts et al. 2008; Jakobsson and Ågren 2014). Habitat loss and fragmentation also negatively affect food resources for pollinators, which are flowering arable weeds. This group of plants may be also breeding sites and larval host plants for these insects. Weeds are vanishing in intensively managed agricultural landscapes (Andreasen et al. 1996; Hyvönen et al. 2003; Chamorro et al. 2016) that homogenize and simplify plant-pollination network, speeding up the decline of diversity of pollinating insects (Baude et al. 2016).
Agricultural landscapes play numerous functions for humans apart from food production. For example, in fields, there may be built windmills, solar panels, silos, and other structures. These man-made structures are frequently described as negatively affecting local biota, but surprisingly, some may also attract or even play an important role for numerous species (e.g., electricity pylons, see: Tryjanowski et al. 2013; Rosin et al. 2016a). These marginal habitats, often regarded as “novel ecosystems” (Hobbs et al. 2009; Tropek et al. 2013; Moroń et al. 2014), are a unique combination of environmental features that do not usually exist in nature. Such specific conditions may cause the formation of new species assemblages (Lundholm and Richardson 2010; Lenda et al. 2012). There is growing evidence that highly transformed and degraded patches, like quarries, railway embankments, or road verges, can paradoxically become a surrogate habitat for many species including those of high conservation status, when their natural habitats disappear in agricultural landscapes (Beneš et al. 2003; Heneberg et al. 2012; Lenda et al. 2012; Moroń et al. 2014, 2017; Berg et al. 2016).
Increasingly popular anthropogenic elements in farmland are wind farms which produce carbon-free energy although their positive effect on the environment is controversial (Rosin et al. 2016b). For example, in Poland, the first windmill was built in 1991; in 2011, the installed power was 1800 MW, but at the end of 2015, the power grew to 4886 MW (Central Statistical Office of Poland 2016). Now in Poland, there are 1193 windmills with a total capacity 5807 MW.Footnote 1 The most discussed issue on wind farm is bird and bat collisions with turbines (Rydell et al. 2010; Rosin et al. 2016b). There are also other problems like noise generated by turbines and alteration to the microclimate of adjacent fields including increased temperature of the ground, modification of humidity of the air at ground level, and heat transfer between the ground and atmosphere (Roy 2011; Walsh-Thomas et al. 2012).
However, windmills may also benefit some of the farmland species as they provide new microhabitats and specific vegetation in the homogenized landscape dominated by crops. Around the windmill, there is a part of unused space spontaneously overgrown by flowering weeds (Supplementary material 1, Figs. S1 and S2). This suggests that windmills may provide surrogate habitats for some wild species including plants and pollinating insects. The aim of this study is to assess the role of windmill site in enhancing biodiversity of weeds and pollinating insects in a homogeneous landscape. We compared species composition in three habitats: areas around windmills (called simply “windmills” throughout the paper), in the grassland patches (typical habitat for pollinating insects; Abrol 2012), and in adjacent arable fields to assess potential contribution of each habitat to species richness, abundance, and diversity of weeds and pollinating insects. To our knowledge, there is currently no study analyzing the importance of windmill structures for sustaining biodiversity.
Materials and methods
Study area
The study was conducted from May to August 2014 at a wind farm in Gołańcz (52° 56′ 38″ N, 17° 17′ 58″ E) in a typical homogeneous agricultural landscape of western Poland. The whole district is covered in about 76% by arable land, while forests occupy only 15% of the district area (Central Statistical Office of Poland 2013).
This wind farm consists of 53 windmills, each with nominal power of 1.5 MW and height equal up to 120 m.Footnote 2 Windmills are loosely dispersed on arable fields within an area of 48.5 km2. The construction of windmills involves the transformation of relief including the elimination of existing form of land use. Usually at each windmill, there is a graveled access square with approximate dimensions 20 × 30 m used by power station service (Supplementary material 1, Figs. S1 and S2). The edge of this square and the area around the tower of windmill are overgrown by segetal vegetation. The studied landscape includes also seminatural grasslands, mainly Arrhenatheretum elatioris mesotrophic communities or purple moorgrass meadow Molinietalia caeruleae (Supplementary material 1, Fig. S3) with patchy distribution and single or double mowing during vegetation period.
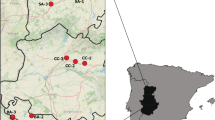
We selected 10 windmills and 10 control grasslands as study sites (Fig. 1). Each site was surrounded by field crops (14 cereal crops and 6 rape fields). Cereal and rape fields were equally divided between windmills and grasslands. Since there were no differences in pollinator diversity and abundance between two types of crops (pollinators H′ diversity index: generalized linear mixed model (GLMM) F 1, 35 = 0.064, p = 0.802; total pollinators species GLMM F 1, 37 = 0.021, p = 0.885; total pollinators abundance GLMM F 1, 37 = 0.646, p = 0.421) nor did we find differences in pollinators diversity and abundance between fields adjacent to windmill and fields bordering with grasslands (pollinators H′ diversity index: t 16.74 = − 0.596, p = 0.559; total pollinators species: t 17.32 = 0.062, p = 0.952; total pollinators abundance: t 14.72 = − 0.663, p = 0.518), we treated these fields as one habitat type hereafter referred to as “field” (see “Data analysis and statistics”). Control grasslands were small patches of a mean size (± SD) equalling 1.0 ± 0.87 ha (min = 0.14 ha; max = 2.76 ha), separated from each other by an average distance of 2021 ± 1493 m (min = 285 m; max = 4575 m). Sampled windmills were separated from each other by 1103 ± 1014 m (min = 244 m; max = 2942 m). The mean distance between two closest plots (windmill or grassland) was 835 ± 765 m (min = 154 m; max = 3579 m).
Fieldworks were conducted during clear weather with low wind speeds (up to 3 on the Beaufort scale), minimum air temperature of 17 °C, and cloud cover up to 30%. Windmills occasionally operated during surveys depending on the wind power. Insects were caught between 9:00 a.m. and 4 p.m. during three surveys (first—May/June [rape flowering period]; second—early July; third—end of July). At each windmill, we marked four sampling points: one in the center of the square and three near its edge between the square and the field (Fig. S1 in Supplementary material 1). Four consecutive points were located along a transect in the field every 15 m from the square edge into the field (Fig. S1 in Supplementary material 1). Within the grassland patches, we have distributed four points in a similar way to windmills encompassing the area of similar size as the windmill site and determining the next four points along a field transect. In total, within a study area, we had 160 sampling points, and on each of them there were three counts, giving a total of 480 samples (Fig. S1 in Supplementary material 1). The insects were caught in an entomological net, and each count consisted of 50 sweeps of net in a 2-m radius (approximate sampling time 1 min). Threatened species of bumblebees were determined in place and released. The study material was kept in 70% ethanol until the taxonomic revision. The insects (bees, butterflies, and flies) were pinned and then determined according to species. Within each sampling point, we recorded data on vegetation in a radius of 2 m, including the total percentage cover and the list of plant species with percentage cover of each species.
Due to generally the small number of insects, we pooled data from sampling points within each site (for each windmill, grassland, and field).
Data analysis and statistics
To evaluate differences in plant and pollinator species richness, abundance, and Shannon H′ diversity index between habitats, we used GLMMs with Poisson error and log-link function (or Gaussian error distribution and identity link in case of the diversity index and plant cover) built in R version 3.3.2 (R Core Team 2016) using the “lme4” package (Bates et al. 2015). The fixed categorical variable was habitat type (three levels: windmill, grassland, field). As a random factor, we included a site identity (a pair windmill-field or grassland-field). We identified statistically significant differences between levels of the habitat type via paired contrasts in GLMMs (Quinn and Keough 2002). In each model, we included two covariates: the distance to the nearest grassland patch (DistanceG) and distance to the nearest windmill (DistanceW) to control for the effect of spatial arrangement of these habitats in a landscape. We also allowed the interaction terms between habitat type (three levels: windmill, grassland, field) and DistanceG as well as habitat type and DistanceW to control for possibly different responses of species groups to the proximity of grasslands and windmills in different habitat types. However, if any interaction term was statistically nonsignificant, it was removed from a final model (Bolker et al. 2008). DistanceG and DistanceW were log-transformed to avoid impact of detached observations (Quinn and Keough 2002). We did these analyses separately for bees (in two ways: with and without honeybee), butterflies, and flies. We also checked if the spatial autocorrelation affected the results of the generalized linear mixed models by calculating Moran I statistics for models’ residuals (Dormann et al. 2007) in R package “pgrimes” (Giraudoux 2017). However, there was no indication of spatial autocorrelation in the dependent variables (Fig. S4 in Supplementary material 1). To analyze how windmills influence species composition of pollinators and plants in a landscape, we conducted canonical correspondence analysis (CCA) with plot identity as supplementary variable using Canoco 5 (Lepš and Šmilauer 2003). We estimated an impact of each explanatory variable to ordination by calculating pseudo-F statistics with p values given after the Bonferroni correction. This analysis was also conducted separately for the three pollinator groups studied. Co-correspondence analysis (CoCa) was used to test if plant species composition in windmills correlate with species composition of all pollinating insects (ter Braak and Schaffers 2004).
Results
In the study period, we captured a total of 299 individuals including 137 flies, 87 butterflies, and 75 bees (Table 1). The most abundant species was honeybee (15% of total material, 60% of all bees). The most species rich group was Diptera order, represented mainly by Syrphidae family; 70% of all captured butterflies belonged to Pieridae family. Three hundred and twenty-five of the 480 samples contained no pollinator. In one site (a field), we did not catch any pollinating insect. Within all study plots, we noted 134 plant species. The list of all pollinators and plant species is available in Supplementary material 1 (Tables S1 and S2).
Effect of windmills on total numbers of pollinators
The studied habitats significantly differ in pollinator diversity, species richness, and abundance (pollinators H′ diversity index GLMM1 F 2, 34 = 33.32, p < 0.001; total pollinators species GLMM2 F 2, 35 = 22.39, p < 0.001; total pollinators abundance GLMM3 F 2, 36 = 37.59, p < 0.001). For all those three response variables, fields have lower values than windmill sites and grasslands (Table 2). However, there were no significant differences in pollinators diversity and abundance between windmill sites and grasslands (Table 2, Fig. 2a–c). Species diversity index at windmills (but not in grassland patches or fields) increased with the distance to the nearest windmill (Table 2, Fig. 3a). Pollinator species richness increased with the distance to the nearest grassland patch (Table 2, Fig. 3b).
The relationship between habitat type and pollinator Shannon diversity H′ index (a), total number of pollinators species per plot (b), and pollinator abundance within a plot (c). Points represent means estimated in generalized linear mixed models. Error bars show 0.95% confidence level also derived from generalized linear mixed models
Canonical correspondence analysis revealed that there were significant differences in pollinator species composition among habitat types (test of first ordination axis: pseudo-F = 2.5, p = 0.002; test of all axes: pseudo-F = 1.9, p = 0.002). The first two axes explained 9.5% of variation in species composition. Variables that statistically contributed to the ordination were windmill sites (pseudo-F = 1.9, p adj. = 0.006) and grasslands (pseudo-F = 2.6, p adj. = 0.006) which accounted for 4.8 and 6.5% of the variation in the pollinator species data, respectively (Fig. 4a).
Effect of windmills on plants
There were differences in plant species H′ diversity index, species richness, and cover among habitats (plants H′ diversity index GLMM4 F 2, 35 = 268.62, p < 0.001; total plants species GLMM5 F 2, 36 = 61.545, p < 0.001; plant cover GLMM6 F 2, 35 = 370.2, p < 0.001). Plants H′ diversity index was lower on grasslands and fields than at windmills (Table 3, Fig. 5a). The total number of plant species was lower on fields than on windmill sites and grasslands, while plant cover was significantly higher on grasslands and fields than on windmill sites (Table 3, Fig. 5b, c).
The relationship between habitat type and plant Shannon diversity H′ index (a), total number of plant species (b), and mean plant cover within plots (c). Points represent means estimated in generalized linear mixed models. Error bars show 0.95% confidence level also derived from generalized linear mixed models
The CCA performed for plant communities revealed that they were also significantly different among habitat types (test of the first axis pseudo-F = 3.4, p = 0.002; test of all axes pseudo-F = 3.1, p = 0.002). All three types of habitat had significantly different plants species (windmills pseudo-F = 2.4, p adj. = 0.006; grasslands pseudo-F = 3.4, p adj. = 0.006; fields pseudo-F = 3.1, p adj. = 0.006). Windmill sites and grasslands explained 5.8 and 8.1% of variation in plant species composition, respectively, while fields explained 7.5% of variation (Fig. 4b). The first two CCA axes explained 14.2% of variation in species composition.
Finally, co-correspondence analysis was performed to assess the covariance between pollinator and plant communities. The first two axes of CoCa analysis explained 33.8% of variation in the pollinators and plants data. The test of all axes showed a significant relation between pollinator and plant assemblages (trace = 3.71, p = 0.04, Fig. 6a, b).
Individual pollinator group response to windmills
Habitat associations for particular groups of pollinators were described by GLMMs summarized in Table S3 in Supplementary material 1. Bees had the highest H′ diversity index, species richness, and abundance at windmills, while grassland and fields had significantly less species and individuals (Fig. S5a–c in Supplementary material 1). After removing the honeybee from the analysis, windmills had higher species diversity index and species richness than grasslands and fields (Table S3, Fig. S5d, e). The number of wild bee individuals at windmills was higher than in fields but not statistically different from grasslands (Table S3, Fig. S5f in Supplementary material 1). Bees also responded to the proximity of other grassland patches and windmills (Table S3). Bee species richness (with or without honeybee) and wild bee abundance increased with the distance to the nearest grassland patch (Table S3, Fig. S6a–c in Supplementary material 1). Moreover, wild bee species diversity index at windmills (but not grasslands) decreased with distance to the nearest windmill (Table S3, Fig. S6d).
For butterflies, there were significantly higher species H′ diversity index, species richness, and abundance within grasslands than at windmills and in fields; however, windmills had substantially more butterfly individuals than fields (Fig. S7 in Supplementary material 1). Butterfly abundance decreased with the distance to the nearest windmill regardless of habitat type (Table S3).
For flies, the effect of windmills was similar as for all pollinators pooled: fields had a lower H′ diversity index, species richness, and abundance than windmills or grasslands and with no differences between the latter two (Fig. S8 in Supplementary material 1).
Species composition of particular pollinator groups was related to habitat type in butterflies (test of first axis pseudo-F = 2.9, p = 0.002; test of all axes pseudo-F = 1.6, p = 0.018) and flies (test of first axis pseudo-F = 2.1, p = 0.002; test of all axes pseudo-F = 1.7, p = 0.002, Fig. S9b, c), but not in bees (test on first axis pseudo-F = 1.5, p = 0.242; test on all axes pseudo-F = 1.1, p = 0.294, Fig. S9a). The exclusion of the honeybee from the analysis did not alter the results of the ordination for bees (test on first axis pseudo-F = 1.2, p = 0.362; test on all axes pseudo-F = 1.1, p = 0.284, Fig. S9d). The first two CCA axes explained 12.8% of variation in the composition of butterflies composition and 11.2% of the composition of flies. Grasslands and windmill sites contributed to community differentiation in butterflies and flies, but not in bees. Detailed tests on the effects of the explanatory variables are summarized in Table S4 in Supplementary material 1.
Discussion
Habitats for pollinators and plants
We demonstrated that windmill sites supported equal species richness, diversity, and abundance of pollinating insects as their typical habitat—seminatural grassland patches and higher than species-poor crop fields. Moreover, species composition was different in windmills, grasslands, and fields, indicating that windmills contribute to species diversity at the landscape scale in the study area. Not only populations of pollinating insects were supported by windmills. Weed species richness and diversity was the highest at windmills. As in pollinators, each of the three habitat types was characterized by a different plant species composition. These results have important implications for the functioning and conservation of plant-pollinator network in a homogeneous farmland.
Marginal habitats (some of them being also novel ecosystems) such as balks, fallows, ditches, road verges, and field borders may enable maintaining species diversity when there is a lack of natural habitats (Jankowiak and Ławicki 2014; Moroń et al. 2014; Piekarska-Boniecka et al. 2015; Assandri et al. 2016). Many analyses paid attention to the role of these marginal habitats in spatial dynamics of insects and insect-pollinated plants (Banaszak 1992; Raemakers et al. 2001; Ricketts et al. 2008; Jakobsson and Ågren 2014). In homogeneous landscapes, these marginal areas may be a habitat surrogate crucial for the survival of populations of endangered species. Such areas function often as movement and dispersal corridors or stepping stones in inhospitable matrix as pollinators rely on food and nesting sources that have patchy distribution (Kajzer-Bonk et al. 2016; Moroń et al. 2017). The positive effect of proximity of other windmills on wild bee diversity index and butterfly abundance indicates that at least for some species windmills may increase population connectivity in a landscape. Nevertheless, it would be interesting to investigate the influence of windmill network on pollinator movements and pollen propagation across the landscape. What is unique for windmills is that they are predominantly arranged in rows and connected with roads, thus may act as stepping stones enhancing permeability of a landscape for pollinators.
High pollinator diversity and species richness even at isolated windmills indicates that these sites may act as a biodiversity hot spot inside extensive fields. It remains unclear if these marginal habitats may exist as independent and self-sufficient units as there are studies showing that the amount of supported biodiversity depends on the proximity of seminatural grassland habitats that function as a population source (Öckinger and Smith 2007; Jauker et al. 2009; Lenda and Skórka 2010). However, in our study area, windmills that were more distant from grasslands had more pollinating species (especially bees) and species composition at windmills was different from that in grassland plots. This suggests that windmills may be independent habitat patches able to sustain local populations and specific species communities. Another plausible explanation of different pollinator species composition among habitats is species filtering at early stage of succession (Lebrija-Trejos et al. 2010).
In forming species-rich communities of pollinating insects, often human disturbance and spontaneous succession of early stage vegetation play a role. Evidences show that the growing vegetation provides a source of food, but with the progress of succession, the availability of bare ground becomes limited (Tropek et al. 2010, 2016). This is the reason why sometimes conservation approaches involving the ban of any management lead to vegetation overgrowth and disappearance of some ground-nesting species. In this way, many natural reserves in central Europe which used to comprise high diversity of plants, bees, and wasps in the 1940s and 1950s now are lacking several ground-dwelling species of pollinators (Kosior et al. 2007; Tropek et al. 2010). The same situation applies to reclamation of postindustrial areas (Heneberg et al. 2012). A limited percentage cover of vegetation around windmills may also be a factor supporting the colonization of these areas by bees since it is known that some ground-nesting species prefer places with bare ground (Cane 1991). Other features, like the presence of stony ground, slopes, and heaps of excavated soil under a windmill, might also be important.
The high value of plant diversity H′ index at windmills indicates that these plots are characterized by a large variety of species and equability of cover of each species while grasslands are rather dominated by grass species with lower cover of flowering dicotyledons. The pollinators are dependent on plants, including weeds, and the stability of pollination networks and services requires the simultaneous protection of floral diversity and pollinators (Rollin et al. 2016). In our study plots, we examined plants as potential food base, but little is known about nesting opportunities for these insects provided by plants. The strong dependence of the pollinator community on plant communities revealed by CoCA analysis suggests that both food and nesting sites may play a role. Moreover, windmills may be a safer place for nesting than grasslands and fields due to limited management (mowing was made once about mid-August in studied windmills). However, there are several threats to plants and pollinators at windmills such as spraying with herbicides and pesticides (a common practice on the graveled windmill squares) or some maintenance earthworks, e.g., melioration. Therefore, future research in the wind turbine landscapes should focus on estimation nest site availability, nest site preferences and reproduction of selected species, and alleviation of potential threats.
Despite that we demonstrated a general positive impact of windmills on pollinator diversity, there were differences in responses between taxonomical groups of pollinators. Butterflies were the most specialized pollinators that were found to inhabit mostly grasslands, probably because grasses with flowering plants may support many species at different life stages (Settele et al. 2009). There was an interesting result for bees as they were significantly more diverse and abundant around a wind turbine, but their communities did not differ significantly between the analyzed habitats. The analysis conducted with the exclusion of domesticated honeybees also suggested the superior value of the windmill area for wild bees. Social bees in particular require appropriate nesting site with proximity of floral sources to feed the larvae and these two needs are best met in habitats around the turbine. In turn, the semimoist grasslands in our study site are more likely to be used by hygrophilous species of flies than by xerophilous bees (Bańkowska 1980). Although different species have different habitats and food requirements at various life stages, some species were closely associated with windmill sites and we can state that these structures participate in increasing the diversity of these groups at the local and landscape scales.
As bees and butterflies responded in different ways to windmills, flies turn out to be the most widely spread group of insects. Adult syrphids are often mentioned as important food-specialized pollinators (Ssymank et al. 2008; Biesmeijer et al. 2006), but they are given less attention in the literature (Larson et al. 2001). As other pollinators such as honeybees or bumblebees cannot effectively support pollination in all crops and wild plants alone (Potts et al. 2010), therefore, sustaining a high diversity of plant species composition requires participation of both generalist and specialist pollinators (Fontaine et al. 2005). Thus, the role of flies in maintaining plant-pollinator network should be investigated more thoroughly in future studies.
There are also other groups of animals that could potentially use windmill sites (Dudek et al. 2015). For example, small mammals were found by Łopucki and Mróz (2016) to occupy wind farms with similar abundance and species composition as control sites. However, there are also groups of animals such as birds that may be highly negatively affected by windmills (Rosin et al. 2016b); thus, the potential value of windmills for sustaining local species diversity should also consider different responses of different taxonomic groups to windmills.
Practical recommendations
The ability of pollinators and plants to occupy small habitat patches is highlighted as conservational advantage that makes the possibility of implementation of various relatively simple conservation actions (Cane 2001). Our results allow us to propose several practices that should focus on ensuring floral diversity and pollinator nesting availabilities. Under windmills, pollinator food plants can be planted which should be native to this region and should be selected basing on the flowering period to provide food for pollinators during the entire season (as a guide, see Vaughan et al. 2007). Appropriate supervision should also prevent the area against overgrowth and maintaining an appropriate share of bare soil because such initial stages of vegetation succession usually have the highest wild plant species diversity (Tscharntke et al. 2011). Some studies have shown the positive effect of installing artificial nests on local populations of pollinators (Dicks et al. 2010). These actions may be introduced both at the stage of windmill construction and at already existing windmills. On the other hand, the negative effects related to service and maintaining of the windmills can also be taken into account. Activities such as the use of pesticides and intensive mowing and some earthworks or influx of pollutants from neighboring fields can harm pollinators and destroy their nests (Barmaz et al. 2012, Krupke et al. 2012). Thus, it is demanded to work out optimal management schemes that minimize the negative impact of these factors. For example, lower herbicide use near windmills could be advised. It is also important to raise public awareness of values of windmill sites, even if many of them may be perceived as ruderal and visually unattractive.
Conclusion
In total, our study depicts the area of the wind farm as a potentially valuable habitat for wild plants and pollinating insects, organisms that decline in a farmland. However, we do not discuss studies that show negative effect of wind energy on other groups of animals, but we highlight that every enterprise may have also positive sides which may compensate or balance other threats. Thus, evaluation of effects of environmental changes on biodiversity should take into account different local processes. A lot of efforts are put into the creation of protected areas, while many anthropogenic and postindustrial sites conceal unexplored potential for sustaining biodiversity. This paper underlines the value of marginal, small habitats for biodiversity conservation that may alleviate negative effects of wind farm development and environmental conflicts in the anthropogenically modified landscapes.
Notes
Map of renewable energy sources in Poland: http://www.ure.gov.pl/uremapoze/mapa.html (accessed 1 February 2017)
Wind farm developer website, nontechnical executive summary: http://www.edpr.com/sustainability/documents-library-and-publications/ (accessed 1 February 2017)
References
Abrol DP (2012) Pollination biology: biodiversity conservation and agricultural production. Springer, Dordrecht
Andreasen C, Stryhn H, Streibig J (1996) Decline of the flora in Danish arable fields. J Appl Ecol 33:619. https://doi.org/10.2307/2404990
Assandri G, Bogliani G, Pedrini P, Brambilla M (2016) Diversity in the monotony? Habitat traits and management practices shape avian communities in intensive vineyards. Agric Ecosyst Environ 223:250–260. https://doi.org/10.1016/j.agee.2016.03.014
Banaszak J (1992) Strategy for conservation of wild bees in an agricultural landscape. Agric Ecosyst Environ 40:179–192. https://doi.org/10.1016/0167-8809(92)90091-o
Bańkowska R (1980) Fly communities of the family Syrphidae in natural and anthropogenic habitats of Poland. Mem Zool 33:3–93
Barmaz S, Vaj C, Ippolito A, Vighi M (2012) Exposure of pollinators to plant protection products. Ecotoxicology 21:2177–2185. https://doi.org/10.1007/s10646-012-0971-7
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01
Baude M, Kunin WE, Boatman ND, Conyers S, Davies N, Gillespie MAK, Morton RD, Smart SM, Memmott J (2016) Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530:85–88. https://doi.org/10.1038/nature16532
Beneš J, Kepka P, Konvička M (2003) Limestone quarries as refuges for European xerophilous butterflies. Conserv Biol 17:1058–1069. https://doi.org/10.1046/j.1523-1739.2003.02092.x
Berg Å, Bergman K, Wissman J, Żmihorski M, Öckinger E (2016) Power-line corridors as source habitat for butterflies in forest landscapes. Biol Conserv 201:320–326. https://doi.org/10.1016/j.biocon.2016.07.034
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. https://doi.org/10.1126/science.1127863
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Cane JH (1991) Soils of ground-nesting bees (Hymenoptera: Apoidea): texture, moisture, cell depth and climate. J Kansas Entomol Soc 64:406–413. https://doi.org/10.2307/25085307
Cane JH (2001) Habitat fragmentation and native bees: a premature verdict? Conserv Ecol 5. https://doi.org/10.5751/es-00265-050103
Central Statistical Office of Poland (2013) Rural areas in Poland—National Agricultural Census 2010. Warsaw
Central Statistical Office of Poland (2016) Energy from renewable sources in 2015. Warsaw
Chamorro L, Masalles R, Sans F (2016) Arable weed decline in Northeast Spain: does organic farming recover functional biodiversity? Agric Ecosyst Environ 223:1–9. https://doi.org/10.1016/j.agee.2015.11.027
Dicks L, Showler D, Sutherland W (2010) Bee conservation: evidence for the effects of interventions (vol. 1). 1st ed. Pelagic Publishing, Exeter. 10.14411/eje.2011.026
Dormann CF, McPherson JM, Araujo MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling DW, Kühn I, Ohlemüller R, Peres-Neto RR, Reineking B, Schröder B, Schurr FM, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628. https://doi.org/10.1111/j.2007.0906-7590.05171.x
Dudek K, Dudek M, Tryjanowski P (2015) Wind turbines as overwintering sites attractive to an invasive lady beetle, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Coleopts Bull 69:665–669. https://doi.org/10.1649/0010-065x-69.4.665
Fontaine C, Dajoz I, Meriguet J, Loreau M (2005) Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4(1):e1. https://doi.org/10.1371/journal.pbio.0040001
Giraudoux P (2017) pgirmess: data analysis in ecology. R package version 1.6.7. https://CRAN.R-project.org/package=pgirmess)
Green RE, Cornell SJ, Scharlemann JPW, Balmford A (2005) Farming and the fate of wild nature. Science 307:550–555. https://doi.org/10.1126/science.1106049
Heneberg P, Bogusch P, Řehounek J (2012) Sandpits provide critical refuge for bees and wasps (Hymenoptera: Apocrita). J Insect Conserv 17:473–490. https://doi.org/10.1007/s10841-012-9529-5
Hobbs R, Higgs E, Harris J (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 24:599–605. https://doi.org/10.1016/j.tree.2009.05.012
Hyvönen T, Ketoja E, Salonen J, Jalli H, Tiainen J (2003) Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agric Ecosyst Environ 97:131–149. https://doi.org/10.1016/s0167-8809(03)00117-8
Jakobsson A, Ågren J (2014) Distance to semi-natural grassland influences seed production of insect-pollinated herbs. Oecologia 175:199–208. https://doi.org/10.1007/s00442-014-2904-z
Jankowiak Ł, Ławicki Ł (2014) Marginal habitats as important refugia for riparian birds during flood years. Bird Study 61:125–129. https://doi.org/10.1080/00063657.2013.874978
Jauker F, Diekötter T, Schwarzbach F, Wolters V (2009) Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc Ecol 24:547–555. https://doi.org/10.1007/s10980-009-9331-2
Kajzer-Bonk J, Skórka P, Nowicki P, Bonk M, Król W, Szpiłyk D, Woyciechowski M (2016) Relative contribution of matrix structure, patch resources and management to the local densities of two large blue butterfly species. PLoS One 11:e0168679. https://doi.org/10.1371/journal.pone.0168679
Kevan P, Viana B (2003) The global decline of pollination services. Biodiversity 4:3–8. https://doi.org/10.1080/14888386.2003.9712703
Kosior A, Celary W, Olejniczak P, Fijal J, Król W, Solarz W, Płonka P (2007) The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 41:79. https://doi.org/10.1017/s0030605307001597
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 1:e29268. https://doi.org/10.1371/journal.pone.0029268
Larson BMH, Kevan PG, Inouye DW (2001) Flies and flowers: taxonomic diversity of anthophiles and pollinators. Can Entomol 133:439–465. https://doi.org/10.4039/ent133439-4
Lebrija-Trejos E, Pérez-García EA, Meave JA, Bongers F, Poorter L (2010) Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 91:386–398. https://doi.org/10.1890/08-1449.1
Lenda M, Skórka P (2010) Patch occupancy, number of individuals and population density of the Marbled White in a changing agricultural landscape. Acta Oecol 36:497–506. https://doi.org/10.1016/j.actao.2010.07.002
Lenda M, Skórka P, Moroń D (2010) Invasive alien plant species—a threat or an opportunity for pollinating insects in agricultural landscapes? In: Lee TH (ed) Agricultural economics: new research. Nova Science Publishers, New York
Lenda M, Skórka P, Moroń D, Rosin Z, Tryjanowski P (2012) The importance of the gravel excavation industry for the conservation of grassland butterflies. Biol Conserv 148:180–190. https://doi.org/10.1016/j.biocon.2012.01.014
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO, 1st edn. Cambridge University Press, New York
Łopucki R, Mróz I (2016) An assessment of non-volant terrestrial vertebrates response to wind farms—a study of small mammals. Environ Monit Assess 188:1–9. https://doi.org/10.1007/s10661-016-5095-8
Lundholm J, Richardson P (2010) Mini-review: Habitat analogues for reconciliation ecology in urban and industrial environments. J Appl Ecol 47:966–975. https://doi.org/10.1111/j.1365-2664.2010.01857.x
Moroń D, Lenda M, Skórka P, Szentgyorgyi H, Settele J, Woyciechowski M (2009) Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscape. Biol Conserv 142:1322–1332. https://doi.org/10.1016/j.biocon.2008.12.036
Moroń D, Skórka P, Lenda M, Rożej-Pabijan E, Wantuch M, Kajzer-Bonk J, Celary W, Mielczarek Ł, Tryjanowski P (2014) Railway embankments as new habitat for pollinators in an agricultural landscape. PLoS One 9:e101297. https://doi.org/10.1371/journal.pone.0101297
Moroń D, Skórka P, Lenda M, Celary W, Tryjanowski P (2017) Railway lines affect spatial turnover of pollinator communities in an agricultural landscape. Divers Distrib 23(9):1090–1097. https://doi.org/10.1111/ddi.12600
Nabhan GP, Buchmann SL (1997) Services provided by pollinators. In: Daily GC (ed) Nature’s services: social dependence on natural ecosystems. Island Press, Washington, pp 133–150
Öckinger E, Smith H (2007) Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J Appl Ecol 44:50–59. https://doi.org/10.1111/j.1365-2664.2006.01250.x
Piekarska-Boniecka H, Mazur R, Wagner A, Trzciński P (2015) Selected elements of cultural landscape structure in Wielkopolska region of Poland as habitats for the parasitoid hymenoptera Pimplinae (Hymenoptera, Ichneumonidae). Insect Conserv Diver 8:54–70. https://doi.org/10.1111/icad.12082
Pimentel D, Stachow U, Takacs DA, Brubaker HW, Dumas AR, Meaney JJ, O’Neil JAS, Onsi DE, Corzilius DB (1992) Conserving biological diversity in agricultural/forestry systems. Bioscience 42:354–362. https://doi.org/10.2307/1311782
Potts S, Biesmeijer J, Kremen C, Neumann P, Schweiger O, Kunin W (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Available at http://www.R-project.org/
Rader R, Bartomeus I, Garibaldi L, Garratt M, Howlett B, Winfree R, Cunningham S, Mayfield M, Arthur A, Andersson G, Bommarco R, Brittain C, Carvalheiro L, Chacoff N, Entling M, Foully B, Freitas B, Gemmill-Herren B, Ghazoul J, Griffin S, Gross C, Herbertsson L, Herzog F, Hipólito J, Jaggar S, Jauker F, Klein A, Kleijn D, Krishnan S, Lemos C, Lindström S, Mandelik Y, Monteiro V, Nelson W, Nilsson L, Pattemore D, de O Pereira N, Pisanty G, Potts S, Reemer M, Rundlöf M, Sheffield C, Scheper J, Schüepp C, Smith H, Stanley D, Stout J, Szentgyörgyi H, Taki H, Vergara C, Viana B, Woyciechowski M (2016) Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci U S A 113:146–151. https://doi.org/10.1073/pnas.1517092112
Raemakers IP, Schaffers AP, Sýkora KV, Heijerman T (2001) The importance of plant communities in road verges as a habitat for insects. Proc Exper Appl Entomol Netherlands Entomol Soc 12:101–106
Ricketts T, Regetz J, Steffan-Dewenter I, Cunningham S, Kremen C, Bogdanski A, Gemmill-Herren B, Greenleaf S, Klein A, Mayfield M, Morandin L, Ochieng’ A, Viana B (2008) Landscape effects on crop pollination services: are there general patterns? Ecol Lett 11:499–515. https://doi.org/10.1111/j.1461-0248.2008.01157.x
Rollin O, Benelli G, Benvenuti S, Decourtye A, Wratten S, Canale A, Desneux N (2016) Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review. Agron Sustain Dev 36:1–22. https://doi.org/10.1007/s13593-015-0342-x
Rosin Z, Skórka P, Pärt T, Żmihorski M, Ekner-Grzyb A, Kwieciński Z, Tryjanowski P (2016a) Villages and their old farmsteads are hot spots of bird diversity in agricultural landscapes. J Appl Ecol 53:1363–1372. https://doi.org/10.1111/1365-2664.12715
Rosin Z, Skórka P, Szymański P, Tobolka M, Luczak A, Tryjanowski P (2016b) Constant and seasonal drivers of bird communities in a wind farm: implications for conservation. PeerJ 4:e2105. https://doi.org/10.7717/peerj.2105
Roy SB (2011) Simulating impacts of wind farms on local hydrometeorology. J Wind Eng Ind Aerod 99:491–498. https://doi.org/10.1016/j.jweia.2010.12.013
Rydell J, Bach L, Dubourg-Savage M, Green M, Rodrigues L, Hedenström A (2010) Bat mortality at wind turbines in northwestern Europe. Acta Chiropterol 12:261–274. https://doi.org/10.3161/150811010x537846
Scherr SJ, McNeely JA (2008) Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes. Philos Trans R Soc Lond Ser B Biol Sci 363:477–494. https://doi.org/10.1098/rstb.2007.2165
Sekercioglu CH, Boyce MS, Tscharntke T, Davidar P, Kremen C (2010) Ecosystem functions and services. In: Sodhi NS, Ehrlich PR (eds) Conservation biology for all. Oxford University Press, New York, pp 45–72. https://doi.org/10.1093/acprof:oso/9780199554232.003.0004
Settele J, Steiner R, Reinhardt R, Feldmann R (2009) Schmetterlinge – Die Tagfalter Deutschlands (2nd ed). Eugen, Ulmer Verlag, Stuttgart
Söderström B, Svensson B, Vessby K, Glimskär A (2001) Plants, insects and birds in semi-natural pastures in relation to local habitat and landscape factors. Biodivers Conserv 10:1839–1863. https://doi.org/10.1023/A:1013153427422
Ssymank A, Kearns C, Pape T, Thompson F (2008) Pollinating flies (Diptera): a major contribution to plant diversity and agricultural production. Biodiversity 9:86–89. https://doi.org/10.1080/14888386.2008.9712892
Stoate C, Báldi A, Beja P, Boatman N, Herzon I, van Doorn A, de Snoo G, Rakosy L, Ramwell C (2009) Ecological impacts of early 21st century agricultural change in Europe—a review. J Environ Manag 91:22–46. https://doi.org/10.1016/j.jenvman.2009.07.005
ter Braak C, Schaffers A (2004) Co-correspondence analysis: a new ordination method to relate two community compositions. Ecology 85:834–846. https://doi.org/10.1890/03-0021
Tropek R, Kadlec T, Karesova P, Spitzer L, Kocarek P, Malenovsky I, Banar P, Tuf I, Hejda M, Konvicka M (2010) Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J Appl Ecol 47:139–147. https://doi.org/10.1111/j.1365-2664.2009.01746.x
Tropek R, Cerna I, Straka J, Cizek O, Konvicka M (2013) Is coal combustion the last chance for vanishing insects of inland drift sand dunes in Europe? Biol Conserv 162:60–64. https://doi.org/10.1016/j.biocon.2013.03.027
Tropek R, Cerna I, Straka J, Kocarek P, Malenovsky I, Tichanek F, Sebek P (2016) In search for a compromise between biodiversity conservation and human health protection in restoration of fly ash deposits: effect of anti-dust treatments on five groups of arthropods. Environ Sci Pollut Res 23:13653–13660. https://doi.org/10.1007/s11356-015-4382-1
Tryjanowski P, Sparks T, Jerzak L, Rosin Z, Skórka P (2013) A paradox for conservation: electricity pylons may benefit avian diversity in intensive farmland. Conserv Lett 7:34–40. https://doi.org/10.1111/conl.12022
Tscharntke T, Batáry P, Dormann CF (2011) Set-aside management: how do succession, sowing patterns and landscape context affect biodiversity? Agric Ecosyst Environ 143:37–44. https://doi.org/10.1016/j.agee.2010.11.025
Vaughan M, Shepard M, Kremen C, Black SH (2007) Farming for bees: guidelines for providing native bee habitat on farms. The Xerces Society, Portland Available at http://www.xerces.org/pubs_merch/Farming_for_Bees.htm
Walsh-Thomas J, Cervone G, Agouris P, Manca G (2012) Further evidence of impacts of large-scale wind farms on land surface temperature. Renew Sust Energ Rev 16:6432–6437. https://doi.org/10.1016/j.rser.2012.07.004
Acknowledgements
We thank Michał Żmihorski for helpful comments on the manuscript.
Funding
This work was supported by the Institute of Nature Conservation, Polish Academy of Sciences (Kraków, Poland) through the Institute’s statutory funds and through grant funding for PhD students and young scientists given from the Institute of Botany, Polish Academy of Sciences (Kraków, Poland).
Author information
Authors and Affiliations
Contributions
PS and PT conceived the idea and designed the methodology; SP collected the data; WBC, PS, and ŁEM determined the species; and SP and PS analyzed the data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 2845 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pustkowiak, S., Banaszak-Cibicka, W., Mielczarek, Ł.E. et al. The association of windmills with conservation of pollinating insects and wild plants in homogeneous farmland of western Poland. Environ Sci Pollut Res 25, 6273–6284 (2018). https://doi.org/10.1007/s11356-017-0864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0864-7