Abstract
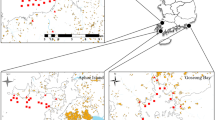
Levels of Escherichia coli and male-specific bacteriophages (MSBs) were determined in the filter feeders obtained from retail markets, commercial farms, and wild beds in Korea. The accumulation and elimination of E. coli and MSBs were compared between ascidians and bivalves (oysters and mussels) during relaying and depuration. E. coli concentrations in ascidians from retail markets ranged between < 20 and 460 most probable number/100 g while MSBs were not detected. E. coli levels in bivalves from commercial farms and wild beds were not significantly different but bacterial levels in ascidians were consistently lower. Ascidians exhibited much lower ability than bivalves to accumulate E. coli and MSBs during relaying in a polluted coastal area. This study also shows that an equilibrium was developed between levels of microbes in water and ascidians and shellfish during relaying. E. coli and MSBs in ascidians decreased quickly during depuration in a clean seawater tank. However, after 1 day, E. coli in bivalves decreased by only 1.1–1.6 logs, and the elimination of MSBs was negligible. Therefore, depuration is an effective means to reduce the health risk of contaminated ascidians.



Similar content being viewed by others
References
APHA (American Public Health Association) (1970) Recommended procedures for the examination of seawater and shellfish. American Public Health Association, Washington DC
Bedford AJ, Williams G, Bellamy AR (1978) Virus accumulation by the rock oyster Crassostrea glomerata. Appl Environ Microbiol 35(6):1012–1018
Berry R, Younger A (2009) Interspecies comparison of E. coli accumulation in bivalve shellfish using data obtained from official control monitoring under EU Regulation 854/2004. Presented at the 7th International Conference on Molluscan Shellfish Safety (ICMSS09 Nantes, France
Bone Q, Carré C, Chang P (2003) Tunicate feeding filters. J Mar Biol Ass U K 83:907–919
Burkhardt W, Calci KR (2000) Selective accumulation may account for shellfish-associated viral illness. Appl Environ Microbiol 66(4):1375–1378. https://doi.org/10.1128/AEM.66.4.1375-1378.2000
Burkhardt W, Rippey SR, Watkins WD (1992a) Depuration rates of northern quahogs Mercenaria mercenaria and eastern oysters Crassostrea virginica in ozone- and ultraviolet light-disinfected seawater systems. J Shellfish Res 11:105–109
Burkhardt W, Watkins WD, Rippey SR (1992b) Survival and replication of male-specific bacteriophages in molluscan shellfish. Appl Environ Microbiol 58(4):1371–1373
Cabelli VJ, Heffernan WP (1970) Elimination of bacteria by the soft shell clam Mya arenaria. J Fish Res Board Canada 27(9):1579–1587. https://doi.org/10.1139/f70-179
DePaola A, Jones JL, Woods J, Burkhardt W, Calci KR, Krantz JA, Bowers JC, Kasturi K, Byars RH, Jacobs E, Williams-Hill D, Nabe K (2010) Bacterial and viral pathogens in live oysters: 2007 United States market survey. Appl Environ Microbiol 76(9):2754–2768. https://doi.org/10.1128/AEM.02590-09
Doré WJ, Lees DN (1995) Behavior of Escherichia coli and male-specific bacteriophage in environmentally contaminated bivalve molluscs before and after depuration. Appl Environ Microbiol 61(8):2830–2834
Doré WJ, Mackie M, Lees DN (2003) Levels of male-specific RNA bacteriophage and Escherichia coli in molluscan bivalve shellfish from commercial harvesting areas. Lett Appl Microbiol 36(2):92–96. https://doi.org/10.1046/j.1472-765X.2003.01268.x
EC (European Commission) (2005) Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R2073:20071227:EN:PDF. Accessed 22 June 2016
Edberg SC, Rice EW, Karlin RJ, Allen MJ (2000) Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol 88(S1):106S–116S. https://doi.org/10.1111/j.1365-2672.2000.tb05338.x
Galbraith HS, Frazier SE, Allison B, Vaughn CC (2009) Comparison of gill surface morphology across a guild of suspension-feeding unionid bivalves. J Molluscan Stud 75(2):103–107. https://doi.org/10.1093/mollus/eyn045
Ha KS, Yoo HD, Shim KB, Kim JH, Lee TS, Kim PH, Ju JY, Lee HJ (2011) Evaluation of the influence of inland pollution sources on shellfish growing areas after rainfall events in Geoje Bay, Korea. Korean J Fish Aquat Sci 44:612–621
ISO (International Organization for Standardization) (2015) ISO 16649-3:2015, Microbiology of the food chain—horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—part 3: detection and most probable number technique using 5-bromo-4-chloro-3-indolyl-beta-D-glucuronide. International Organization for Standardization, Geneva
Iwamoto M, Ayers T, Mahon BE, Swerdlow DL (2010) Epidemiology of seafood-associated infections in the United States. Clin Microbiol Rev 23(2):399–411. https://doi.org/10.1128/CMR.00059-09
Kershaw S, Campos CJA, Reese A, Mitchard N, Kay D, Wyer M (2013) Impact of chronic microbial pollution on shellfish. Cefas, Lowestoft, UK
KMFDS (Korea Ministry of Food and Drug Safety) (2016) Korea food code http://fse.foodnara.go.kr/residue/RS/jsp/menu_02_01_01.jsp. Accessed 22 June 2016
Kryger J, Riisgard HU (1988) Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia 77(1):34–38. https://doi.org/10.1007/BF00380921
Le Guyader FS, Loisy F, Atmar RL, Hutson AM, Estes MK, Ruvoën-Clouet N, Pommepuy M, Le Pendu J (2006) Norwalk virus-specific binding to oyster digestive tissues. Emerg Infect Dis 12(6):931–936. https://doi.org/10.3201/eid1206.051519
Lees D, Younger A, Doré B (2010) Depuration and relaying. In: Rees G, Pond K, Kay D, Bartram J, Santo Domingo J (eds) Safe management of shellfish and harvest waters. World Health Organization, IWA Publishing, London, pp 145–181
Mcleod C, Hay B, Grant C, Greening G, Day D (2009) Inactivation and elimination of human enteric viruses by Pacific oysters. J Appl Microbiol 107(6):1809–1818. https://doi.org/10.1111/j.1365-2672.2009.04373.x
Mizuta S, Isobe S, Yoshinaka R (2002) Existence of two molecular species of collagen in the muscle layer of the ascidian (Halocynthia roretzi). Food Chem 79(1):9–13. https://doi.org/10.1016/S0308-8146(02)00167-X
Mok JS, Lee KJ, Kim PH, Lee TS, Lee HJ, Jung YJ, Kim JH (2016a) Bacteriological quality evaluation of seawater and oysters from the Jaranman-Saryangdo area, a designated shellfish growing area in Korea: impact of inland pollution sources. Mar Pollut Bull 108(1–2):147–154. https://doi.org/10.1016/j.marpolbul.2016.04.036
Mok JS, Lee TS, Kim PH, Lee HJ, Ha KS, Shim KB, Lee KJ, Jung YJ, Kim JH (2016b) Bacteriological quality evaluation of seawater and oysters from the Hansan-Geojeman area in Korea, 2011–2013: impact of inland pollution sources. SpringerPlus 5(1):1412. https://doi.org/10.1186/s40064-016-3049-9
NZFSA (New Zealand Food Safety Authority) (2006) Animal products (Specifications for bivalve molluscan shellfish). http://www.foodsafety.govt.nz/elibrary/industry/Animal_Products-Applies_Anyone.pdf. Accessed 22 June 2016
Oliveira J, Cunha A, Castilho F, Romalde JL, Pereira MJ (2011) Microbial contamination and purification of bivalve shellfish: crucial aspects in monitoring and future perspectives—a mini-review. Food Control 22(6):805–816. https://doi.org/10.1016/j.foodcont.2010.11.032
Pawiro S (2010) Bivalves: global production and trade trends. In: Rees G, Pond K, Kay D, Bartram J, Santo Domingo J (eds) Safe management of shellfish and harvest waters. World Health Organization, IWA Publishing, London, pp 11–19
Petersen JK (2007) Ascidian suspension feeding. J Exp Mar Biol Ecol 342(1):127–137. https://doi.org/10.1016/j.jembe.2006.10.023
Petersen JK, Svane I (2002) Filtration rate in seven Scandinavian ascidians: implications of the morphology of the gill sac. Mar Biol 140:397–402
Potasman I, Paz A, Odeh M (2002) Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clin Infect Dis 35(8):921–928. https://doi.org/10.1086/342330
Power UF, Collins JK (1989) Differential depuration of poliovirus, Escherichia coli, and a coliphage by the common mussel, Mytilus edulis. Appl Environ Microbiol 55(6):1386–1390
Randløv A, Riisgård HU (1979) Efficiency of particle retention and filtration rate in four species of ascidians. Mar Ecol Prog Ser 1:55–59. https://doi.org/10.3354/meps001055
Ribes M, Coma R, Gili JM (1998) Seasonal variation of in situ feeding rates by the temperate ascidian Halocynthia papillosa. Mar Ecol Prog Ser 175:201–213. https://doi.org/10.3354/meps175201
Riisgård HU, Larsen PS (2010) Particle capture mechanisms in suspension-feeding invertebrates. Mar Ecol Prog Ser 418:255–293. https://doi.org/10.3354/meps08755
Rippey SR (1994) Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev 7(4):419–425. https://doi.org/10.1128/CMR.7.4.419
Sair AI, D'Souza DH, Jaykus LA (2002) Human enteric viruses as causes of foodborne disease. Compr Rev Food Sci Food Saf 1:72–89
Stuart V, Klumpp DW (1984) Evidence for food-resource partitioning by kelp-bed filter feeders. Mar Ecol Prog Ser 16:27–37. https://doi.org/10.3354/meps016027
Timoney JF, Abston A (1984) Accumulation and elimination of Escherichia coli and Salmonella typhimurium by hard clams in an in vitro system. Appl Environ Microbiol 47(5):986–988
U.S. EPA (Environmental Protection Agency) (2001) Method 1601: Male-specific (F+) and Somatic Coliphage in Water by Two-step Enrichment Procedure. EPA 821-R-01-030, US EPA, Washington DC
U.S. FDA (Food and Drug Administration) (2015) National Shellfish Sanitation Program (NSSP), NSSP Guide for the Control of Molluscan Shellfish. http://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.htm. Accessed 20 April 2017
Ueki Y, Shoji M, Suto A, Tanabe T, Okimura Y, Kikuchi Y, Saito N, Sano D, Omura T (2007) Persistence of caliciviruses in artificially contaminated oysters during depuration. Appl Environ Microbiol 73(17):5698–5701. https://doi.org/10.1128/AEM.00290-07
Vaughn CC, Hakenkamp CC (2001) The functional role of burrowing bivalves in freshwater ecosystems. Freshw Biol 46(11):1431–1446. https://doi.org/10.1046/j.1365-2427.2001.00771.x
Yang JH, Mok JS, Jung YJ, Lee KJ, Kwon JY, Park K, Moon SY, Kwon SJ, Ryu AR, Lee TS (2017) Distribution and antimicrobial susceptibility of Vibrio species associated with zooplankton in coastal area of Korea. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.07.054
Younger AD, Reese RA (2013) Comparison of Escherichia coli levels between bivalve mollusc species across harvesting sites in England and Wales. J Shellfish Res 32(2):527–532. https://doi.org/10.2983/035.032.0232
Funding
This work was supported by a grant from the National Institute of Fisheries Science in Korea (R2017057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kim, J.H., Shim, K.B., Shin, S.B. et al. Comparison of bioaccumulation and elimination of Escherichia coli and male-specific bacteriophages by ascidians and bivalves. Environ Sci Pollut Res 24, 28268–28276 (2017). https://doi.org/10.1007/s11356-017-0736-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0736-1