Abstract
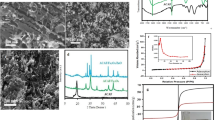
Activated carbon (AC)/CoFe2O4 nanocomposites, MAC-1 and MAC-2, were prepared by a simple pyrolytic method using a mixture of iron(III)/cobalt(II) benzoates and iron(III)/cobalt(II) oxalates, respectively, and were used as efficient adsorbents for the removal of amoxicillin (AMX) and paracetamol (PCT) of aqueous effluents. The synthesized nanocomposites were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), vibrating sample magnetometry (VSM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX) and transmission electron microscopy (TEM). The sizes of cobalt ferrite nanoparticles formed from benzoates of iron(III)/cobalt(II) and oxalates of iron(III)/cobalt(II) precursors were in the ranges of 5–80 and 6–27 nm, respectively. The saturation magnetization (M s), remanence (M r) and coercivity (H c) of the MAC-2 nanocomposites were found to be 3.07 emu g−1, 1.36 emu g−1 and 762.49 Oe; for MAC-1, they were 0.2989 emu g−1, 0.0466 emu g−1 and 456.82 Oe. The adsorption kinetics and isotherm studies were investigated, and the results showed that the as-prepared nanocomposites MAC-1 and MAC-2 could be utilized as an efficient, magnetically separable adsorbent for environmental cleanup. The maximum sorption capacities obtained were 280.9 and 444.2 mg g−1 of AMX for MAC-1 and MAC-2, respectively, and 215.1 and 399.9 mg g−1 of PCT using MAC-1 and MAC-2, respectively. Both adsorbents were successfully used for simulated hospital effluents, removing at least 93.00 and 96.77% for MAC-1 and MAC-2, respectively, of a mixture of nine pharmaceuticals with high concentrations of sugars, organic components and saline concentrations.








Similar content being viewed by others
References
Ai L, Li M, Li L (2011) Adsorption of methylene blue from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: kinetics, isotherms, and thermodynamics. J Chem Eng Data 56:3475–3483
Arya V, Philip L (2016) Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Micropor Mesopor Mater 232:273–280
Barbosa F Jr, Lima EC, Krug FJ (2000) Determination of arsenic in sediment and soil slurries by electrothermal atomic absorption spectrometry using W-Rh permanent modifier. Analyst 125:2079–2083
Bouabi YE, Farahi A, Labjar N, Hajjaji SE, Bakasse M, Mhammedi MAE (2016) Square wave voltammetric determination of paracetamol at chitosan modified carbon paste electrode: application in natural water samples, commercial tablets and human urines. Mat Sci Eng C 58:70–77
Calvete T, Lima EC, Cardoso NF, Dias SLP, Ribeiro ES (2010) Removal of brilliant green dye from aqueous solutions using home made activated carbons. CLEAN—Soil Air Water 38:521–532
Cardoso NF, Lima EC, Royer B, Bach MV, Dotto GL, Pinto LAA, Calvete T (2012) Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J Hazard Mater 241-242:146–153
Chen Y, Man Luo M, Wangfeng Cai W (2016) Influence of operating parameters on the performance of magnetic seeding flocculation. Environ Sci Pollut Res 23:2873–2881
Do MH, Phan NH, Nguyen TD, Pham TTS, Nguyen VK, Vu TTT, Nguyen TKP (2011) Activated carbon/Fe3O4 nanoparticle composite: fabrication, methyl orange removal and regeneration by hydrogen peroxide. Chemosphere 85:1269–1276
dos Reis GS, Sampaio CH, Lima EC, Wilhelm M (2016a) Preparation of novel adsorbents based on combinations of polysiloxanes and sewage sludge to remove pharmaceuticals from aqueous solutions. Colloids Surf A Physicochem Eng Asp 497:304–315
dos Reis GS, Mahbub MKB, Wilhelm M, Lima EC, Sampaio CH, Saucier C, Dias SLP (2016b) Activated carbon from sewage sludge for removal of sodium diclofenac and nimesulide from aqueous solutions. Korean J Chem Eng 33:3149–3161
dos Santos DC, Adebayo MA, Lima EC, Pereira SFP, Cataluña R, Saucier C, Thue PS, Machado FM (2015) Application of carbon composite adsorbents prepared from coffee waste and clay for the removal of reactive dyes from aqueous solutions. J Brazilian Chem Soc 26:924–938
Ehrensberger K, Schmalle H, Oswald H, Reller A (1999) Thermochemical reactivity of transition metal acetates and of a novel DMSO solvate of iron(II) acetate in molecular hydrogen. J Therm Anal Calorim 57:139–149
Eslami A, Asadi A, Meserghani M, Bahrami H (2016) Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J Mol Liq 222:739–744
Estévez MC, Font H, Nichkova M, Salvador JP, Varela B, Baeza FS, Marco MP (2005) Immunochemical determination of pharmaceuticals and personal care products as emerging pollutants. In: Barceló D (ed) Emerging organic pollutants in waste waters and sludge. Springer, Berlin Heidelberg, pp 181–244
Freitas VAA, Maia LA, Belardinelli RE, Ardisson JD, Pereira MC, Oliveira LAC (2016) Magnetic iron species highly dispersed over silica: use as catalysts for removal of pollutants in water. Environ Sci Pollut Res. doi:10.1007/s11356-016-6495-6
Gao C, Li W, Morimoto W, Nagaoka Y, Maekawa T (2006) Magnetic carbon nanotubes: synthesis by electrostatic self-assembly approach and application in biomanipulations. J Phys Chem B 110:7213–7220
Gupta A, Garg A (2015) Utilisation of sewage sludge derived adsorbents for the removal of recalcitrant compounds from wastewater: mechanistic aspects, isotherms, kinetics and thermodynamics. Bioresou Technol 194:214–224
Hermanek M, Zboril R, Mashlan M, Machala L, Schneeweiss O (2006) Thermal behaviour of iron(ii) oxalate dihydrate in the atmosphere of its conversion gases. J Mater Chem 16:1273–1280
Hu D, Wang L (2016) Adsorption of amoxicillin onto quaternized cellulose from flax noil: kinetic, equilibrium and thermodynamic study. J Taiwan Inst Chem Eng 64:227–234
Hu L, Chen W, Xie X, Liu N, Yang Y, Wu H, Yao Y, Pasta M, Alshareef HN, Cui Y (2011) Symmetrical MnO2-carbon nanotube-textile nanostructures for wearable pseudocapacitors with high mass loading. ACS Nano 5:8904–8913
Huang Q, Wang X, Li J, Dai C, Gamboa S, Sebastian PJ (2007) Nickel hydroxide/activated carbon composite electrodes for electrochemical capacitors. J Power Sources 164:425–429
Huiqun C, Meifang Z, Yaogang Y (2006) Decoration of carbon nanotubes with iron oxide. J Solid State Chem 179:1208–1213
Kuyumcu OK, Bayazit SS, Salam MA (2016) Antibiotic amoxicillin removal from aqueous solution using magnetically modified graphene nanoplatelets. J Ind Eng Chem 36:198–205
Kyzas GZ, Lazaridis NK, Bikiaris DN (2013) Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohydr Polym 91:198–208
Li H, Yan Q, Hng HH (2016) Nitrogen doped carbon nanotubes encapsulated MnO nanoparticles derived from metal coordination polymer towards high performance lithium-ion battery anodes. Electrochim Acta 187:406–412
Lima EC, Barbosa-Jr F, Krug FJ, Guaita U (1999) Tungsten-rhodium permanent chemical modifier for lead determination in digests of biological materials and sediments by electrothermal atomic absorption spectrometry. J Anal At Spectrom 14:1601–1605
Lima EC, Barbosa RV, Brasil JL, Santos AHDP (2002) Evaluation of different permanent modifiers for the determination of arsenic, cadmium and lead in environmental samples by electrothermal atomic absorption spectrometry. J Anal At Spectrom 17:1523–1529
Lima EC, Brasil JL, Santos AHDP (2003) Evaluation of Rh, Ir, Ru, W-Rh, W-Ir, and W-Ru as permanent modifiers for the determination of lead in ashes, coals sediments, sludges, soils, and freshwaters by electrothermal atomic absorption spectrometry. Anal Chim Acta 484:233–242
Lima EC, Adebayo MA, Machado FM (2015) Chapter 3—kinetic and equilibrium models of adsorption in carbon nanomaterials as adsorbents for environmental and biological applications, Bergmann, CP, Machado FM, editors, Springer 33–69
Machado FM, Bergmann CP, Lima EC, Royer B, de Souza FE, Jauris IM, Calvete T, Fagan SB (2012) Adsorption of Reactive Blue 4 dye from water solutions by carbon nanotubes: experiment and theory. Phys Chem Chem Phys 14:11139–11153
Mehdinia A, Akbari M, Kayyal TB, Azad M (2015) High-efficient mercury removal from environmental water samples using di-thio grafted on magnetic mesoporous silica nanoparticles. Environ Sci Pollut Res 22:2155–2165
Pezoti O, Cazetta AL, Bedin KC, Souza LS, Martins AC, Silva TL, Santos-Jr O-O, Visentainer JV, Almeida VC (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isothermand thermodynamic studies. Chem Eng J 288:778–778
Podder MS, Majumder CB (2016) Application of granular activated carbon/MnFe2O4 composite immobilized on C. glutamicum MTCC 2745 to remove As(III) and As(V): kinetic, mechanistic and thermodynamic studies. Spectrochim Acta Part A Mol Biomol Spectrosc 153:298–314
Prola LDT, Acayanka E, Lima EC, Umpierres CS, Vaghetti JCP, Santos WO, Laminsi S, Njifon PT (2013) Comparison of Jatropha curcasshells in natural form and treated by non-thermal plasma as biosorbents for removal of Reactive Red 120 textile dye from aqueous solution. Ind Crop Prod 46:328–340
Puchana-Rosero MJ, Lima EC, Ortiz-Monsalve S, Mella B, da Costa D, Poll E, Gutterres M (2016) Fungal biomass as biosorbent for the removal of Acid Blue 161 dye in aqueous solution. Environ Sci Pollut Res. doi:10.1007/s11356-016-8153-4
Purkait MK, Maiti A, DasGupta S, De S (2007) Removal of congo red using activated carbon and its regeneration. J Hazard Mater 145:287–295
Ranjithkumar V, Vairam S (2012) Activated carbon–Mn3O4 nanocomposites—synthesis and magnetic studies. Adv Mater Res 584:182–186
Ranjithkumar V, Hazeen AN, Thamilselvan M, Vairam S (2014a) Magnetic activated carbon-Fe3O4 nanocomposites—synthesis and applications in the removal of acid yellow dye 17 from water. J Nanosci Nanotechnol 14:4949–4959
Ranjithkumar V, Sangeetha S, Vairam S (2014b) Synthesis of magnetic activated carbon/α-Fe2O3 nanocomposite and its application in the removal of acid yellow 17 dye from water. J Hazard Mater 273:127–135
Ribas MC, Adebayo MA, Prola LDT, Lima EC, Cataluña R, Feris LA, Puchana-Rosero MJ, Machado FM, Pavan FA, Calvete T (2014) Comparison of a homemade cocoa shell activated carbon with commercial activated carbon for the removal of reactive violet 5 dye from aqueous solutions. Chem Eng J 248:315–326
Rovani S, Censi MT, Pedrotti-Jr SL, Lima EC, Cataluña R, Fernandes AN (2014) Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. J Hazard Mater 271:311–320
Santos TRT, Silva MF, Nishi L, Vieira MAS, Klein MRF, Andrade MB, Vieira MF, Bergamasco R (2016) Development of a magnetic coagulant based on Moringa oleifera seed extract for water treatment. Environ Sci Pollut Res 23:7692–7700
Sathiya M, Prakash AS, Ramesha K, Tarascon JM, Shukla AK (2011) V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J Am Chem Soc 133:16291–16299
Saucier C, Adebayo MA, Lima EC, Cataluña R, Thue PS, Prola LDT, Puchana-Rosero MJ, Machado FM, Pavan FA, Dotto GL (2015a) Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J Hazard Mater 289:18–27
Saucier C, Adebayo MA, Lima EC, Prola LDT, Thue PS, Umpierres CS, Puchana-Rosero MJ, Machado FM (2015b) Comparison of a homemade Bacury shell activated carbon with MWCNT for the removal of Brilliant Blue FCF food dye from aqueous solutions. Clean Air Soil Water 43:1389–1400
Shan D, Deng S, Zhao T, Wang B, Wang Y, Huang J, Yu G, Winglee J, Wiesner MR (2016) Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J Hazard Mater 305:156–163
Singh AP, Garg P, Alam F, Singh K, Mathur RB, Tandon RP, Chandra A, Dhawan SK (2012) Phenolic resin-based composite sheets filled with mixtures of reduced graphene oxide, γ-Fe2O3 and carbon fibers for excellent electromagnetic interference shielding in the X-band. Carbon 50:3868–3875
Sun CL, Wang CS (2010) Estimation on the intramolecular hydrogen-bonding energies in proteins and peptides by the analytic potential energy function. J Mol Struct 956:38–43
Sun Y, Ji G, Zheng M, Chang X, Zhang Y (2010) Synthesis and magnetic properties of crystalline mesoporous CoFe2O4 with large specific surface area. J Mater Chem 20:945–952
Tan F, Fan X, Zhang G, Zhang F (2007a) Coating and filling of carbon nanotubes with homogeneous magnetic nanoparticles. Mater Lett 61:1805–1808
Tan IAW, Hameed BH, Ahmad AL (2007b) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119
Tang J, Wu S, Wang T, Gong H, Zhang H, Alshehri SM, Ahamad T, Zhou H, Yamauchi Y (2016) Cage-type highly graphitic porous carbon–Co3O4 polyhedron as the cathode of lithium–oxygen batteries. ACS Appl Mater Interfaces 8:2796–2804
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265
Villaescusa I, Fiol N, Poch J, Bianchi A, Bazzicalupi C (2011) Mechanism of paracetamol removal by vegetable wastes: the contribution of π–π interactions, hydrogen bonding and hydrophobic effect. Desalination 270:135–142
Wan S, Bi H, Sun L (2016) Graphene and carbon-based nanomaterials as highly efficient adsorbents for oils and organic solvents. Nanotechnol Rev 5:3–22
Wang H, Qing C, Guo J, Aref AA, Sun D, Wang B, Tang Y (2014) Highly conductive carbon-CoO hybrid nanostructure arrays with enhanced electrochemical performance for asymmetric supercapacitors. J Mater Chem A 2:11776–11783
Yildirim T, Ciraci S (2005) Titanium-decorated carbon nanotubes as a potential high-capacity hydrogen storage medium. Phys Rev Lett 94:175501
Zhang S, Dong Y, Yang Z, Yang W, Wu J, Dong C (2016) Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem Eng J 304:325–334
Zhao H, Liu X, Cao Z, Zhan Y, Shi X, Yang Y, Zhou J, Xu J (2016) Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J Hazard Mater 310:235–245
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq, Brazil) and the Coordination of Improvement of Higher Education Personnel (CAPES, Brazil) for financial support, fellowships, grants and technical support. We also thank Chemaxon for giving an academic research licence for the Marvin Sketch software, version 16.11.1.0, (http://www.chemaxon.com), 2016 used for pharmaceutical physical–chemical properties. We also thank the Centre of Electron Microscopy (CME-UFRGS) for the use of the SEM microscope.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Electronic supplementary material
ESM 1
(PDF 1071 kb)
Rights and permissions
About this article
Cite this article
Saucier, C., Karthickeyan, P., Ranjithkumar, V. et al. Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ Sci Pollut Res 24, 5918–5932 (2017). https://doi.org/10.1007/s11356-016-8304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8304-7