Abstract
Selective catalytic reduction of NO X by hydrogen (H2-SCR) in the presence of oxygen has been investigated over the NiCo2O4 and Pd-doped NiCo2O4 catalysts under varying conditions. The catalysts were prepared by a sol-gel method in the presence of oxygen within 50–350 °C and were characterized using XRD, BET, EDS, XPS, Raman, H2-TPR, and NH3-TPD analysis. The results demonstrated that the doped Pd could improve the catalyst reducibility and change the surface acidity and redox properties, resulting in a higher catalytic performance. The performance of NiCo1.95Pd0.05O4 was consistently better than that of NiCo2O4 within the 150–350 °C range at a gas hourly space velocity (GHSV) of 4800 mL g−1 h−1, with a feed stream containing 1070 ppm NO, 10,700 ppm H2, 2 % O2, and N2 as balance gas. The effects of GHSV, NO/H2 ratios, and O2 feed concentration on the NO conversion over the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts were also investigated. The two samples similarly showed that an increase in GHSV from 4800 to 9600 mL h−1 g−1, the NO/H2 ratio from 1:10 to 1:1, and the O2 content from 0 to 6 % would result in a decrease in NO conversion. In addition, 2 %, 5 %, and 8 % H2O into the feed gas had a slightly negative influence on SCR activity over the two catalysts. The effect of SO2 on the SCR activity indicated that the NiCo1.95Pd0.05O4 possesses better SO2 tolerance than NiCo2O4 catalyst does.

The NiCo1.95Pd0.05O4 catalyst achieved over 90 % NO conversion with N2 selectivity of 100 % in the 200∼250 °C range than the maximum 40.5 % NO conversion over NiCo2O4 with N2 selectivity of approximately 80 % in 350 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen oxides (NO X ), including NO, NO2, N2O, N2O3, N2O4, and N2O5, are mainly derived from fossil fuel combustion (Costa and Efstathiou 2007b). NO X are the major air pollutants that are greatly hazardous to human health and the environment (Xiaoling et al. 2012), causing negative effects such as photochemical smog, acid rain, ozone depletion, ground-level ozone, and greenhouse effects (Qi et al. 2006). A number of techniques have been developed to reduce the emission of NO X . Among them, the selective catalytic reduction of NO X by ammonia (NH3-SCR) is a well-known and widely industrialized NO X control technology for stationary sources such as power plants and nitric acid plants (Li et al. 2010b). Generally, V2O5-WO3 (MO3)/TiO2 is employed as NH3-SCR catalysts (Grossale et al. 2008). However, many problems are encountered in the use of NH3-SCR technology, namely catalyst deterioration, NH3 slip (emissions of unreacted toxic ammonia), ash odor, air heater fouling, and a high running cost (Olympiou and Efstathiou 2011).
H2-SCR has many advantages; for instance, hydrogen as a reductant does not induce any second pollutants and has high activity to reduce NO X efficiently at the lowest possible temperature (Kim and Hong 2010; Machida et al. 2001). Especially for industrial sites where H2 is readily available, H2-SCR is regarded as a possible alternative for NH3-SCR. The catalyst is the most central technology in any H2-SCR process, and its performance directly affects the removal of nitrogen oxides (Koebel et al. 2001; Weirong et al. 2012). Currently, the catalysts of H2-SCR primarily contain supported noble metal oxides (Jun Yub et al. 2003; Yang and Jung 2009), among which the Pt-based and Pd-based catalysts have been revealed to possess good catalytic activity at relatively low temperatures (Chiarello et al. 2007; Costa and Efstathiou 2007a; Qing et al. 2010; Schott et al. 2009). For example, Costa and Efstathiou (2007a) had reported that the Pt/MgO-CeO2 catalyst exhibited a maximum of 95 % NO conversion and 78∼92 % N2 selectivity within the 100–400 °C range. Higher than 90 % NO X conversion within 170–300 °C was presented in the Pd/SiO2 catalyst (Qing et al. 2010). Chiarello et al. (2007) investigated the catalytic reduction of NO X by H2 over Pd-based catalysts with a support consisting of LaCoO3. The 0.5 wt% Pd/LaCoO3 catalyst exhibited a maximum of approximately 100 % NO conversion and over 78 % N2 selectivity at 150 °C.
Nevertheless, noble metals are rare and expensive and they are sensitive to sulfur poisoning. These factors limit their large-scale applications. Therefore, new highly efficient catalysts need to be searched to replace Pt-based and Pd-based catalysts for H2-SCR for NO X . Due to their low price, ready synthesis, and good redox property, transition metal oxides have been widely used as catalysts in various reactions (Auxilia et al. 2014; Chiu et al. 2015). Among them, spinel-type oxides have been widely studied because of their unique structure characteristics, and these oxide catalysts can simultaneously reduce NO X and soot at comparatively low temperatures (possibly within the range typical of diesel exhaust 150–380 °C) (Fino et al. 2008). Chen et al. (2009) had reported that the CuCoOx/TiO2 catalyst exhibited a maximum of 98.9 % NO conversion at 200 °C. Du et al. (2014) had reported that Co3O4 showed high performance on the removal of low-concentration (10 ppm) NO at room temperature.
Nickel cobaltite (NiCo2O4) is a mixed-metal oxide spinel that possesses interesting magnetic properties, rich redox chemistry, good electronic conductivity, and high electrochemical activity (Kim et al. 2000; Rui et al. 2013). In the recent research, NiCo2O4 is usually explored as an electrode material (Chi et al. 2006; Xu et al. 2014) and has rare reports about the catalytic reduction performance in the SCR reaction. Wang et al. (2015b) had reported that NiCo2O4 possessed the highest catalytic activity within 50–400 °C, with NO X conversion of more than 70 % at 150 °C and N2 selectivity of more than 90 % at 100–400 °C. Therefore, we choose nickel cobaltite as a model catalyst.
Generally, doped noble metal can improve the catalytic activity and reduce the operating temperature. Palladium is less expensive and more abundant than platinum (Gaspar and Dieguez 2000), and Pd as an active component shows high activity for H2-SCR and is highly active for H2 activation (Li et al. 2012). The exceptional performance is due to the high dispersion of Pd in the catalyst that forms the Pd-NO intermediates, which adsorb more NO on the catalyst surface and are reduce to N2 by hydrogen with high N2 selectivity (Li et al. 2008). Rodríguez and Saruhan (2010) reported that the highly active centers could be formed by the interaction sites between Pd and supports and high NO X conversion and N2 selectivities could be achieved by the synergistic effects of palladium and perovskites. Moreover, the synergistic effects efficiently enhance H2 temperature-programmed reduction (H2-TPR). Besides, Xu et al. (2015) had researched that doped Pd could make NiFe1.95Pd0.05O4 increase acidity, reducibility, and catalytic activity.
NO was selected as a model nitrogen oxide in the simulated gas (Wang et al. 2009), because nitrogen monoxide accounts for 95–99 % of all nitrogen oxide emissions in flue gas (Fritz and Pitchon 1997). And the Pd-doped nickel cobaltite catalyst was successfully prepared through a sol-gel auto-ignition method and exhibited good catalytic performance for the selective catalytic reduction of NO by H2 in the presence of oxygen at a low temperature. And we have considered the effects of the gas hourly space velocity (GHSV), NO/H2 ratios, and O2 feed concentration on the SCR activity. For practical consideration, we also investigated the durability of the catalyst and its tolerance to SO2 and H2O, respectively.
Materials and methods
Catalyst preparation
The catalyst used in this research was prepared via a sol-gel method using inorganic salts. All of the chemicals were of analytical grade and used without further purification. The compounds were weighed by analytical balance with the molar ratio of citric acid/Ni(NO3)3·6H2O/Co(NO3)3·6H2O/PdCl2 = 3:1:1.95:0.05. Then, these compounds were dissolved in 50 mL deionized water with a concentration of 0.1 mol nitrate precursor (Jauhar et al. 2013). The pH of the mixture solution was additionally adjusted to 5–6 by slowly adding the ammonia solution, then the resulting solution was mixed together under stirring at room temperature for 3 h, and the sol was heated at 80 °C to form a wet gel; afterwards, the gel was dried at 130 °C. After drying, the obtained material was ground into fine powder. In order to obtain crystallized NiCo1.95Pd0.05O4, the powder was calcinated in Muffle furnace at 400 °C for 4 h. Spinel NiCo2O4 was prepared in similar way with the only difference that merely the molar ratio of citric acid/Ni(NO3)3·6H2O/Co(NO3)3·6H2O/PdCl2 was 3:1:2.
According to the drying and roasting process, the particles were easy to reunite and affect the catalyst activity, so about 5 mL polyethylene glycol (PEG) 400 was added to ensure high specific surface area and uniform particle size (Fan and Huang 2011).
Catalyst characterization
The as-prepared products were characterized by powder X-ray diffraction (XRD) using a Rigaku D/Max 2550 diffractometer (Japan) with Cu Kα radiation (λ = 1.54056 Å) operating at 40 kV and 100 mA. The elemental composition of the samples was characterized by energy-dispersive spectrometer (EDS) using a Falion 60s spectrometer. The Brunauer–Emmett–Teller (BET) surface area, average particle size, and pore size of the catalysts were measured with a nitrogen adsorption instrument (Micromeritics, TriStar II 3020) using N2 gas as an adsorbent at the temperature of liquid nitrogen. Prior to BET analysis, the samples were degassed at 300 °C for 3 h.
The X-ray photoelectron spectroscopy (XPS) analysis was conducted in a Quantum 2000 Scanning ESCA Microprobe (Physical Electronics). The instrument uses a focused monochromatic Al Kα X-ray (1486.7 eV) source operated at a 100 W and 100-μm-diameter beam. The binding energy scale was calibrated using the carbon C1s at 284.6 eV for known standards. The de-convolution of XPS peak was performed with the CasaXPS program. The Raman spectroscopy experiments were carried out on an Iuvia microscope instrument (Iuvia Reflerx) with a wavelength of 514.5 nm.
H2-TPR and temperature-programmed desorption of ammonia (NH3-TPD) were performed on AutoChem II 2920 equipped with a thermal conductivity detector (TCD) detector. Prior to the H2-TPR or NH3-TPD analysis, 50 mg of the oven-dried sample was pretreated in a He stream at 300 °C for 60 min to remove the adsorbed H2O and other gases followed by cooling to room temperature. After that, the H2-TPR analysis was performed using a 10 % H2/Ar mixture at a flow rate of 40 mL/min with a heating rate of 10 °C/min to 800 °C, while the NH3-TPD analysis was carried out by 10 % NH3/He mixture with a total flow rate of 40 mL/min at 50 °C for 60 min. After NH3 adsorption, the sample was purged by He (40 mL/min) for another 60 min. The desorption profile was recorded using a TCD by heating the sample to 600 °C at 10 °C/min under a flow of He (50 mL/min).
Catalyst activity testing
The selective catalytic reduction of NO by hydrogen was carried out in a fixed-bed flow micro-reactor, the catalyst bed temperature was controlled by thermocouple which was interpolated into the fixed-bed reactor, and reactor was heated through an Al-518/518P-type artificial intelligence temperature controller. A sample weighed 1 g, and the reactant gas composites consisted of 1071 ppm NO; 1071–10,710 ppm H2 (the concentration was based on the ratio of NO to H2); 0–6 % O2; 2 %, 5 %, and 8 % H2O (when used); 100, 300, and 500 ppm SO2 (when used); and the balance N2. These gases were fed from compressed cylinders provided by Jia Jie Specialty Gases (Shanghai, China) and adjusted with Brooks thermal mass flow controllers. The catalyst was fixed by silica pellets and quartz wool and placed in the constant temperature zone of the tubular reactor. The total flow of the inlet gas and the gas hourly space velocity were based on the change of gas conditions. The gas effluent stream from the reactor was analyzed by a Chemiluminescent NO–NO2–NO X Analyzer (Thermo Scientific, model 42i), and the nitric oxide conversion was indicated using the following equation:
Among them, X NO represents the NO conversion and [NO]inlet and [NO]outlet show the inlet and outlet concentrations of NO in the gas mixture at steady state, respectively.
The Thermo Scientific NO–NO2–NO X Analyzer revealed that the NO X was the sum of NO, NO2, and less low-state nitrogen oxides, and the \( {X}_{{\mathrm{NO}}_X} \) represented the total NO X conversion rate.
where the [NO X ]inlet and [NO X ]outlet show the inlet and outlet concentrations of NO in the gas mixture at steady state, respectively. The N2 selectivity was calculated as follows:
In this equation, the X NO·[NO]inlet expresses the amount of nitric oxide in the transformation, while the \( {X}_{{\mathrm{NO}}_X}\cdot {\left[\mathrm{NO}\right]}_{\mathrm{inlet}} \) expresses the amount of nitrogen from the transformation of nitric oxide.
Results and discussion
Catalyst characterization
Structural and textural properties
The XRD patterns of the samples used in this study are shown in Fig. 1a. The major peaks of the samples at ca. 2θ = 18.9°, 31.1°, 36.7°, 38.4°, 44.6°, 55.4°, 59.1°, and 65.1° could be indexed to (111), (220), (311), (222), (400), (422), (511), and (440) crystal planes of spinel NiCo2O4, respectively. It could be seen that the samples were a spinel cubic structure which were in good agreement with the standard pattern (JCPDS No. 73-1702) (Xu et al. 2014). While the XRD pattern of NiCo1.95Pd0.05O4 was similar to that of NiCo2O4, the diffraction angle theta of (440) crystal planes slightly shifted from 65.1° to 64.9°, which is shown in Fig. 1b. These results indicated that the heavier and larger Pd2+ ions substituted the Co2+ ions in the structure of the nickel cobaltite spinel, resulting in a small angle deviation of XRD peaks (Kavas et al. 2009). And the Pd doping did not cause a significant change in the crystallinity of the samples, possibly due to the palladium that replaced Co without distorting the spinel structure; therefore, the NiCo1.95Pd0.05O4 sample also maintained the spinel cubic structure.
The average crystalline size of NiCo2O4 and NiCo1.95Pd0.05O4 was estimated to be 32.2 and 26.7 nm using Scherrer’s formula based on the (422) peak, respectively (Lou et al. 2008). Scherrer’s formula is as follows:
Among which, D is crystalline size, K is constant, λ is X-ray wavelength (0.154056 nm), β is peak half-high width, and θ is the diffraction angle theta. Due to nickel cobaltite’s spinel cubic structure, K should be changed to 0.943 and a half-high width should be converted into a radian system, [(β / 180) × 3.14].
BET and EDS analysis
The BET surface areas, average particle size, and pore size of the studied NiCo2O4 and NiCo1.95Pd0.05O4 were summarized in Table 1. The surface areas of the NiCo2O4 and NiCo1.95Pd0.05O4 samples were 14.9956 and 12.6566 m2 g−1, respectively, while the average particle sizes were 14.0774 and 15.4802 nm, respectively, which had the reverse order compared to the surface area values. Generally, the larger the particle size, the smaller the BET area. The elemental composition of the samples was investigated using an EDS, as shown in Table 1. The Co/Ni atomic ratio (we chose Ni for comparison due to the stoichiometric amount being 1 in all of the studied samples) for NiCo2O4 was 2.03, which was consistent with the stoichiometric ratio. Due to the palladium doping, the Co/Ni atomic ratio of NiCo1.95Pd0.05O4 was 1.92, deviating slightly from the theoretical ratio of 1.95. The Pd/Ni atomic ratio was 0.049, which was consistent with the theoretical value of 0.05.
H2-TPR and NH3-TPD analysis
In order to investigate the reducibility and acidic properties of the NiCo2O4 and NiCo1.95Pd0.05O4, the as-prepared samples were characterized by H2 temperature-programmed reduction (H2-TPR) and temperature-programmed desorption of ammonia (NH3-TPD) analysis, respectively. As shown in Fig. 2a, there were three distinct reduction peaks in NiCo2O4, and the peaks appeared at 253 and 360 °C, corresponding to the reduction steps of Co3+ to Co2+ and Co2+ to Co0, respectively (Gou et al. 2013; Lim et al. 2015), while the peak appeared at 315 °C, which was attributed to the reduction of Ni2+ to Ni0. However, in comparison to NiCo2O4, the H2-TPR profile of the NiCo1.95Pd0.05O4 catalyst showed one slight peak and one strong broad peak. The slight peak at 129 °C was assigned to the reduction of Pd2+ to Pd0 (Giraudon et al. 2007; Ling et al. 2015), which was observed in pure Ni-Co spinel. And the strong broad peak within the range of 200–350 °C was formed by three reduction peaks fully overlapped, which corresponded to the reductions of Co3+ to Co2+ at 223 °C, Ni2+ to Ni0 at 286 °C, and Co2+ to Co0 at 316 °C. Compared to NiCo2O4, the reduction peaks of NiCo1.95Pd0.05O4 shifted to low temperature, which indicated that the H2 consumption was raised, enhancing the redox properties of catalysts. In addition, the TPR peak areas were an indicator to determine the reducibility of catalyst; the greater the peak area, the stronger the reducibility. The peak areas of H2-TPR were provided in Table 2. It could be seen that the Pd-containing nickel cobaltate exhibited relatively higher TPR areas than NiCo2O4, indicating that the palladium doping could improve the catalyst reducibility.
The acidic properties of the as-prepared samples were shown in Fig. 2b. The NH3 adsorbed on the acid sites and then desorbed at different temperature regions, which was determined by the strength of acid sites and the amount of acid, respectively. The NH3 desorption below 400 °C corresponded to the weak- and medium-strength acid sites (Imran et al. 2013). Nevertheless, the TPD profile of NiCo2O4 showed a broad NH3 desorption peak from 100 to 350 °C, which was assigned to the NH3 desorbed by weak- and medium-strength acid sites. Moreover, the acid amount could be determined by the TPD peak areas and the size of peak area corresponded to the amount of acid. Table 2 showed the NH3-TPD peak areas. Compared to NiCo2O4, NiCo1.95Pd0.05O4 slightly increased the peak areas from 100 to 350 °C, indicating that NiCo1.95Pd0.05O4 showed a relatively more acidic amount than NiCo2O4.
Raman analysis
NiCo2O4 is an inverse spinel with tetragonal (A site) positions occupied by mostly Co3+ and octahedral (B site) positions occupied by nearly equal concentrations of Ni2+ and Co3+ (Iliev et al. 2013). In order to further understand the composition and structural features of the NiCo2O4 and NiCo1.95Pd0.05O4, the samples were characterized with Raman spectroscopy. The typical Raman spectrum was shown in Fig. 3; the peaks of NiCo2O4 at 183, 465, 509, and 651 cm−1 corresponded to F2g, E1g, F2g, and A1g models of NiCo2O4, respectively, and the Co–O and Ni–O stretching vibrations could be detected in the Raman spectrum. These results were well consistent with previously reported literatures (Babu et al. 2014; Li et al. 2015; Liu et al. 2013). Moreover, the peaks at 181, 418, 501, and 644 cm−1 corresponded to F2g, E1g, F2g, and A1g models of NiCo1.95Pd0.05O4, respectively. Compared with that of NiCo2O4, the Raman spectra of the Pd-substituted nickel cobaltate (NiCo1.95Pd0.05O4) exhibited a shift toward lower wave number, which was the result of the heavier and larger Pd2+ ions substituting the Co2+ ions in the structure of the nickel cobaltite spinel.
XPS analysis
In order to investigate the chemical bonding states and compositions of surface elements of as-synthesized NiCo2O4 and NiCo1.95Pd0.05O4, the samples were studied by X-ray photoelectron spectroscopy (XPS), and the results were shown in Fig. 4a–d. The Ni, Co, O, and Pd elements were detected for the prepared samples.
The high-resolution spectrum for the O1s region in Fig. 4a showed two peaks at binding energies of around 529.1 and 530.8 eV, respectively, which had been denoted as O1 (529.1 eV) and O2 (530.8 eV). The O1 at the low energy of 529.1 eV was typical of the O atoms in the O–Co/Ni bonding. And the O2 at 530.8 eV was a characteristic of the as-prepared NiCo2O4 samples, which corresponded to a number of defect sites with low-oxygen coordination in the material with small particle size (Kim et al. 2000; Liu et al. 2013; Rui et al. 2013). The Co2p binding energies and peak shape were similar for the two preparations (Fig. 4b) and yield binding energies of 779.8 and 794.7 eV for the 2p3/2 and 2p1/2 transitions, respectively (Kim et al. 2000). XPS spectra of Co2p3/2 in the two preparations showed two main peaks of binding energies at 779.4 and 781.4 eV, which were assigned to the surface Co3+ and Co2+ species, respectively (Wang et al. 2015b). And this indicated that there were only a few Co2+ species in the octahedral sites and most of the low-spin Co3+ species occupied the octahedral sites (Babu et al. 2014; Kim et al. 2000).
The Ni2p spectra given in Fig. 4c were fitted considering two spin-orbit doublets as a characteristic of Ni2+ and Ni3+ and two shake-up satellites. The fitting peaks at the binding energy of 854.4 and 871.6 eV were indexed to Ni2+, while the fitting peaks at the binding energy of 856.43 and 873.8 eV were ascribed to Ni3+, respectively (Li et al. 2015, 2010a; Yu et al. 2016). The Pd3d spectra, as presented in Fig. 4d, showed two peaks at the binding energy of 337.1 eV for 3d5/2 and 342.5 eV for 3d3/2, respectively. The Pd5/2 binding energy was closed to the value of 336.9 which is a characteristic of Pd2+, indicating that the Pd doped in the as-prepared NiCo1.95Pd0.05O4 sample (Giraudon et al. 2007; Hu et al. 2011; Ling et al. 2015).
These results exhibited that the surface of the as-synthesized NiCo2O4 that contained Ni2+, Ni3+, Co2+, and Co3+ (Moni et al. 2014), while the NiCo1.95Pd0.05O4 also contained Pd2+ except the common elements.
Catalyst performance
SCR activity of the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts
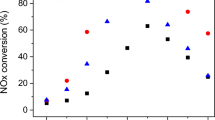
Figure 5 shows the results of the NO conversion and N2 selectivity over the NiCo2O4 and NiCo1.95Pd0.05O4 in the temperature range of 50–350 °C with a GHSV of 4800 mL g−1 h−1 in the presence of 2 % O2. As shown in Fig. 5a, as the reaction temperature increased, the NO conversion firstly increased then decreased over the NiCo1.95Pd0.05O4 catalyst. While the SCR activity over the NiCo2O4 catalyst was always increasing until it reached the maximum NO conversion. The highest NO conversion of the NiCo2O4 catalyst was approximately 40.05 % at 350 °C; yet, the N2 selectivity over the NiCo2O4 catalyst was only 60∼80 %, which is illustrated in Fig. 5b. The NO conversion over the NiCo1.95Pd0.05O4 catalyst was higher than 90 % at a suitable temperature of 200–250 °C with the maximum of 94.65 % at 230 °C. And the N2 selectivity was higher than 90 % in the whole 130–350 °C range, with the maximum closed to 100 % at the 200–270 °C range.
a The effect of the reaction temperature on NO conversion for H2-SCR over the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts in the presence of 2 % O2. b N2 selectivity over the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts in the presence of 2 % O2. c, d The effect of oxygen concentration (0∼6 %) on NO conversion for H2-SCR over the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts. Reaction condition: [NO] = 1070 ppm; [H2] = 10,700 ppm; [O2] = 0 %, 2 % (a, b), 4 %, and 6 %; [N2] = balance; catalyst mass = 1 g; GHSV = 4800 mL g−1 h−1; and T = 50–350 °C. c NiCo2O4. d NiCo1.95Pd0.05O4
Moreover, a previous study showed that the content of O2 was a crucial parameter for the H2-SCR reaction (Yang et al. 2011; Yuan et al. 2013). The combustion of fuel was not sufficient in the old burners, which resulted in the high oxygen content of 6∼12 %. However, the new type of circulating fluidized bed burns the fuel more efficiently, resulting in the low oxygen content of 4∼6 % (Huilin et al. 2000). Hence, in our study, the highest oxygen content was chosen to be 6 %. In general, oxygen can inhibit NO removal efficiency by oxidizing H2 to H2O, but it also oxidizes NO into adsorbed nitrite/nitrate on the surface to enhance the reduction reaction by hydrogen in the H2-SCR process (Machida et al. 2001; Wei et al. 2012). Hence, the effect of the oxygen concentration ([O2] = 0 %, 2 %, 4 %, and 6 %) in the feed gas over the NiCo1.95Pd0.05O4 and NiCo2O4 catalysts was investigated, which also consisted of 1070 ppm NO/10,700 ppm H2/x % O2/N2 at a temperature range of 50–350 °C with a GHSV of 4800 mL g−1 h−1.
The oxygen content in the flue gas from the circulating fluidized bed boiler is generally 4∼6 % (Huilin et al. 2000). Therefore, experiments were performed to examine the O2 tolerance of the prepared catalysts. As illustrated in Fig. 5c, under O2-free reaction conditions, a higher removal efficiency of NO was achieved and an efficiency of 100 % over the NiCo2O4 catalysts was stabilized within a temperature range of 250–350 °C. As the O2 content increased from 0 % to 6 %, the deNO X performance rapidly decreased and the maximum NO conversion decreased from 100 to 30.77 % at the 350 °C. As shown in Fig. 5d, the high NO conversion of 100 % over NiCo1.95Pd0.05O4 was stabilized within a wider temperature range of 150–350 °C in the absence of oxygen, indicating that the doped Pd improved the catalytic activity and shifted the reaction temperature of the efficiency of 100 % from 250 to 150 °C. When the O2 increased to 2 %, the maximum NO conversion over NiCo1.95Pd0.05O4 only changed to 94 % at the 230 °C; as the O2 content increased, the SCR still exhibited good activity for NO reduction, with the maximum NO conversion of 81.16 % with 4 % O2 at 230 °C and 61 % in the presence of 6 % O2 at 230 °C, proving that Pd incorporation not only significantly improves the catalytic activity but also increases the O2 tolerance ability (Wei et al. 2012).
To sum up, the NiCo1.95Pd0.05O4 catalyst showed higher catalytic performance as well as greatly reduced the reaction temperature of the maximum removal efficiency. Hence, the NiCo1.95Pd0.05O4 catalyst was suitable for the selective catalytic reduction of NO by H2 in the presence of oxygen at a low temperature. Therefore, Pd played a very important role in the nickel cobaltite catalyst in the H2-SCR reaction. In addition, palladium did not destroy the spinel structure of NiCo2O4 when it doped in nickel cobaltite, which was consistent with the XRD results. And from the H2-TPR and NH3-TPD activity tests, the NiCo1.95Pd0.05O4 exhibited relatively high TPR areas, reduction level, and slightly larger acidity than NiCo2O4. All these factors resulted in the better catalytic performance of NiCo1.95Pd0.05O4.
Effect of GHSV and NO/H2 ratio
In general, GHSV could significantly affect the NO conversion rate at low temperature, and it was believed that it has less effect on the conversion rate at high temperature (Qi et al. 2006). Consequently, the H2-SCR activity of the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts at different GHSVs (from 4800 to 9300 mL h−1 g−1) and NO and H2 feed concentration ratios (NO/H2 = 1:10–1:1) were investigated at a temperature range of 50–350 °C in the presence of 2 % O2, and the results are shown in Fig. 6.
Effect of gas hourly space velocity and NO/H2 ratios on NO conversion for H2-SCR over the NiCo1.95Pd0.05O4 and NiCo2O4 catalysts. Reaction condition: [H2] = 10,700 ppm (when examined gas hourly space velocity), [NO] = 1070 ppm, [O2] = 2 %, [N2] = balance, catalyst mass = 1 g, GHSV = 4800 mL g−1 h−1 (when NO/H2 ratios were examined), and T = 50–350 °C. a NiCo2O4. b NiCo1.95Pd0.05O4. c NiCo2O4. d NiCo1.95Pd0.05O4
As shown in Fig. 6a, the NO conversion over the NiCo2O4 catalyst decreased with the increasing GHSV. When the GHSV was 4800 mL h−1 g−1, the maximum NO conversion was up to 40.5 % at 350 °C, while when the GHSV increased to 6960 and 9300 mL h−1 g−1, the maximum conversion decreased to 35 % and 31 %, respectively. However, in contrast with NiCo2O4, the NO conversion faintly decreased over the NiCo1.95Pd0.05O4 catalyst with the increase of the GHSV from 4800 to 9600 mL h−1 g−1 at the reaction temperature of 125–350 °C, which was shown in Fig. 6b. When the GHSV was 4800 mL h−1 g−1, the maximum NO conversion was up to 94 % at 230 °C, and when the GHSV was 9300 mL h−1 g−1, the maximum conversion was approximately 92 % at 230 °C.
These results demonstrated that the GHSV was a crucial parameter for the H2-SCR reaction and the space velocity determined the residence time of the gas in the catalyst. As GHSV increased, the residence time of feed gas decreased. The NiCo1.95Pd0.05O4 catalyst showed high NO conversion with the increasing GHSV, due to the Pd addition, which enhanced the redox properties of the active catalyst component. And this was consistent with H2-TPR. Although the increase of GHSV resulted in the decrease of the reaction gas residence time in the catalyst, the activity of the active component was enhanced in the Pd-doped catalyst, resulting in more reduction reaction sites for NO and only a slight decrease of NO conversion. Therefore, the Pd-doped NiCo1.95Pd0.05O4 catalyst showed better catalytic performance than the NiCo2O4 catalyst at different GHSVs (from 4800 to 9300 mL h−1 g−1).
The variation trend of NO conversion over the sample catalysts was roughly similar as the increase of reaction temperature at different NO/H2 ratios, the NO conversion firstly increased then decreased over the NiCo1.95Pd0.05O4 catalyst with the increasing reaction temperature, but the removal of NO over the NiCo2O4 catalyst increased as the temperature increased. As shown in Fig. 6c, the NO conversion over the NiCo2O4 catalyst decreased as the NO and H2 feed concentration ratio increased within a temperature range of 170–350 °C in the presence of 2 % O2. When the NO/H2 ratio was 1:10, the maximum NO conversion was 41 %. When the NO/H2 ratio was changed to 1:5 and 1:1, the NO conversion rate was 34 % and 31 %, respectively, exhibiting that higher H2 concentration that resulted in higher NO conversion. Nevertheless, compared with the NiCo2O4 catalyst, the NiCo1.95Pd0.05O4 catalyst showed higher catalytic activity at different NO and H2 ratios, which is shown in Fig. 6d. When the NO/H2 ratio was 1:10, the maximum NO conversion was more than 95 % at a reaction temperature of 230 °C. Actually, the economic factors must be considered. Industrially, the lower NO/H2 ratio was used to obtain a relatively high NO conversion. When the NO/H2 ratio was changed to 1:5 and 1:1, the NO conversion rate was 76 % and 37 %, respectively. Therefore, the doped Pd largely improved the catalytic activity and NiCo1.95Pd0.05O4 exhibited higher catalytic performance than NiCo2O4 in the common reaction condition.
Effect of the presence of H2O and SO2
H2O and SO2 were usually contained in the industrial flue gases, which could cause a deactivation on SCR catalysts, and the general content of water was 2∼10 %. At the same time, the SCR catalysts are sensitive to sulfur poisoning since sulfur compounds could deposit on the active sites of catalysts and deactivate them irreversibly (Chang et al. 2013; Lee et al. 2013; Yin et al. 2015). Therefore, it was important to investigate the effect of H2O and SO2 on NO conversion over selected catalysts.
The effect of H2O and SO2 on the selective catalytic reduction of nitric oxide with hydrogen over the NiCo2O4 and NiCo1.95Pd0.05O4 catalyst was demonstrated in Fig. 7. As shown in Fig. 7a, when 2 %, 5 %, and 8 % H2O was introduced to feed gas, the NO conversion of NiCo2O4 decreased rapidly from 36.8 %, 36.5 %, and 36.3 % to 33.2 %, 32.5 %, and 31.8 %, respectively, and then the NO conversion recovered slowly to 34.2 %, 33.4 %, and 32.6 % after the removal of H2O, respectively. Compared with the initial value, the decrement of NO conversion was 7.07 %, 8.49 %, and 12.95 %, respectively. It could be seen that the conversion decreased more as the content of H2O increased, which was due to the water-irreversible dissociative adsorption on the active sites of the catalyst (Burch and Coleman 1999; Leicht et al. 2012). And the NO conversion recovered a little, which was due to the reversible competitive adsorption by water. Hence, the influence of water on the catalyst was both reversible and irreversible.
Effect of H2O ([H2O] = 2 %, 5 %, and 8 %) and SO2 ([SO2] = 100, 300, and 500 ppm) on NO conversion for H2-SCR over the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts. Reaction condition: [NO] = 930 ppm (950 ppm, when SO2 was added), [H2] = 9300 ppm (9500 ppm, when SO2 was added), [O2] = 2 %, [N2] = balance, catalyst mass = 1 g, GHSV = 9300 mL g−1 h−1, and flow rate = 155 mL/min. a NiCo2O4, T = 350 °C, when H2O was added. b NiCo1.95Pd0.05O4, T = 230 °C, when H2O was added. c NiCo2O4, T = 350 °C, when SO2 was added. d NiCo1.95Pd0.05O4, T = 230 °C, when SO2 was added
However, the behavior of H2O poisoning of the NiCo1.95Pd0.05O4 catalyst was quite different. As shown in Fig. 7b, after 2 %, 5 %, and 8 % H2O was introduced into the inlet gas, the NO conversion showed a slight decrease approximately from 87.2 % to 86.5 %, 84.5 %, and 80.5 %, respectively, which probably was due to the H2O competitive adsorption with NO as well as with H2. The water on the catalyst could form the additional surface hydroxyls because of the dissociative adsorption of water (Kijlstra et al. 1996), and the surface hydroxyls would neutralize the acid sites, resulting in the reduction of SCR activity. For the Pd-doped catalyst, the surface acidity was enhanced. The multi-acid neutralized the hydroxyls and decreased the water poisoning on active sites of the catalyst. Therefore, the NiCo1.95Pd0.05O4 catalyst has a better H2O tolerance than NiCo2O4. Furthermore, the influence of surface hydroxyls neutralizing the acid sites was irreversible; when the H2O was off, the acidity of acid sites could not recover, and hence, the NO conversion over the NiCo1.95Pd0.05O4 catalyst could not restore to the original level, which was consistent with Fig. 7b.
Moreover, the SO2 tolerance and regenerability of NiCo2O4 and NiCo1.95Pd0.05O4 catalysts were also examined. As shown in Fig. 7c, when the 100 ppm SO2 was added to the reactant gas, the NO conversion of NiCo2O4 at 350 °C decreased rapidly from 35.8 % to 31.6 % in 150 min and only recovered to 32.6 % after the removal of SO2, manifesting the inhibition effect of SO2 on the SCR activity over NiCo2O4 (Chang et al. 2013; Yin et al. 2015). When the SO2 increased to 300 and 500 ppm, the NO conversion decreased rapidly to lower than 27.5 % and 14.5 % and only recovered to 30.6 % and 21.6 % after the removal of SO2, respectively. The decrease of NO conversion was mainly due to the blocking of the active sites by the formation of metal sulfates and/or sulfites (Li et al. 2011). The sulfated species formed on the catalytic center inhibited the SCR activity, which resulted in the decrease of NO conversion. After the supply of SO2 was cut off, the NO conversion recovered slowly in a certain amount, which was mainly due to the regeneration of the part of sulfated catalysts by the hydrogen (Wang et al. 2015a).
The SO2 poisoning behavior to the NiCo1.95Pd0.05O4 catalyst at 230 °C was quite similar. As shown in Fig. 7d, after 100, 300, and 500 ppm SO2 was introduced to the inlet gas, the NO conversion of NiCo1.95Pd0.05O4 decreased steadily from 88.1 % to 83.8 %, 80.5 %, and 51.6 % in 130 min, respectively. The decrement of NO conversion was 4.88 %, 8.63 %, and 41.43 % compared to the initial value; however, the decrement value of NiCo2O4 was 11.73 %, 23.18 %, and 59.50 %, respectively. This was attributed to the improvement of catalytic activity by enhanced surface acidities and redox properties, which was consistent with NH3-TPD and H2-TPR. After 100, 300, and 500 ppm SO2 was off, the NO conversion of NiCo1.95Pd0.05O4 could efficiently recover to 84.4 %, 82.8 %, and 65.5 %, respectively, indicating that the deactivation was partially reversible.
These results demonstrated that the NiCo1.95Pd0.05O4 catalyst possesses better SO2 tolerance than NiCo2O4 catalyst does.
Conclusions
In this study, the NiCo2O4 and NiCo1.95Pd0.05O4 catalysts were investigated for NO selective reduction by hydrogen in the presence of O2. The results showed that the NiCo2O4 catalyst had a certain effect for NO removal, while the Pd-containing NiCo1.95Pd0.05O4 catalyst exhibited better SCR activity with the maximum NO conversion of 94.65 % at 230 °C and the N2 selectivity of 100 %. Moreover, the prepared NiCo1.95Pd0.05O4 exhibited higher H2O and SO2 tolerance than NiCo2O4.
References
Auxilia FM, Ishihara S, Mandal S, Tanabe T, Saravanan G, Ramesh GV, Umezawa N, Hara T, Xu Y, Hishita S (2014) Low-temperature remediation of NO catalyzed by interleaved CuO nanoplates. Adv Mater 26:4481–4485
Babu GA, Ravi G, Hayakawa Y (2014) Surfactant assisted growth and optical studies of NiCo2O4 nanostructures through microwave heating method. Int J Sci Eng Appl 1:17–20
Burch R, Coleman MD (1999) An investigation of the NO/H2/O2 reaction on noble-metal catalysts at low temperatures under lean-burn conditions. Appl Catal B Environ 23:115–121
Chang H, Li J, Yuan J, Chen L, Dai Y, Arandiyan H, Xu J, Hao J (2013) Ge, Mn-doped CeO2–WO3 catalysts for NH3–SCR of NOx: effects of SO2 and H2 regeneration. Catal Today 201:139–144
Chen X, Zhang JF, Huang Y, Tong ZQ, Huang M (2009) Catalytic reduction of nitric oxide with carbon monoxide on copper-cobalt oxides supported on nano-titanium dioxide. J Environ Sci 21:1296–1301
Chi B, Lin H, Li J, Wang N, Yang J (2006) Comparison of three preparation methods of NiCo2O4 electrodes. Int J Hydrog Energy 31:1210–1214
Chiarello GL, Ferri D, Grunwaldt JD, Forni L, Baiker A (2007) Flame-synthesized LaCoO3-supported Pd: 2. Catalytic behavior in the reduction of NO by H2 under lean conditions. J Catal 252:137–147
Chiu CH, Hsi HC, Lin HP (2015) Multipollutant control of Hg/SO2/NO from coal-combustion flue gases using transition metal oxide-impregnated SCR catalysts. Catal Today 245:2–9
Costa CN, Efstathiou AM (2007a) Low-temperature H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst. Appl Catal B Environ 72:240–252
Costa CN, Efstathiou AM (2007b) Mechanistic aspects of the H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst. J Phys Chem C 111:3010–3020
Du Y, Huang W, Hua Z, Wang Y, Cui X, Wu M, Shu Z, Zhang L, Wang J, Chen H (2014) A facile ultrasonic process for the preparation of Co3O4 nanoflowers for room-temperature removal of low-concentration NOx. Catal Commun 57:73–77
Fan J, Huang W (2011) Preparation of Cu-Zn-Al bifunctional catalyst by sol-gel method with the assistance of PEG and its catalytic performance. Chin J Catal 32:139–143
Fino D, Russo N, Saracco G, Specchia V (2008) Removal of NOx and diesel soot over catalytic traps based on spinel-type oxides. Powder Technol 180:74–78
Fritz A, Pitchon V (1997) The current state of research on automotive lean NOx catalysis. Appl Catal B Environ 13:1–25
Gaspar AB, Dieguez LC (2000) Dispersion stability and methylcyclopentane hydrogenolysis in Pd/Al 2 O 3 catalysts. Appl Catal A Gen 201:241–251
Giraudon JM, Elhachimi A, Wyrwalski F, Siffert S, Aboukaïs A, Lamonier JF, Leclercq G (2007) Studies of the activation process over Pd perovskite-type oxides used for catalytic oxidation of toluene. Appl Catal B Environ 75:157–166
Gou Y, Liang X, Chen B (2013) Porous Ni–Co bimetal oxides nanosheets and catalytic properties for CO oxidation. J Alloys Compd 574:181–187
Grossale A, Nova I, Tronconi E (2008) Study of a Fe–zeolite-based system as NH3-SCR catalyst for diesel exhaust after treatment. Catal Today 136:18–27
Hu L, Peng Q, Li Y (2011) Low-temperature CH4 catalytic combustion over Pd catalyst supported on Co3O4 nanocrystals with well-defined crystal planes. ChemCatChem 3:868–874
Huilin L, Guangbo Z, Rushan B, Yongjin C, Gidaspow D (2000) A coal combustion model for circulating fluidized bed boilers. Fuel 79:165–172
Iliev MN, Silwal P, Loukya B, Datta R, Kim DH, Todorov ND, Pachauri N, Gupta A (2013) Raman studies of cation distribution and thermal stability of epitaxial spinel NiCo2O4 films. J Appl Phys 114:033514–033514-5
Imran M, Kim DH, Al-Masry WA, Mahmood A, Hassan A, Haider S, Ramay SM (2013) Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis. Polym Degrad Stab 98:904–915
Jauhar S, Goyal A, Lakshmi N, Chandra K, Singhal S (2013) Doping effect of Cr3+ ions on the structural, magnetic and electrical properties of Co–Cd ferrites: a study on the redistribution of cations in CoCd0.4CrxFe1.6−xO4 (0.1 ≤ x ≤ 0.6) ferrites. Mater Chem Phys 139:836–843
Jun Yub L, Bum KS, Sung Chang H (2003) Characterization and reactivity of natural manganese ore catalysts in the selective catalytic oxidation of ammonia to nitrogen. Chemosphere 50:1115–1122
Kavas H, Baykal A, Toprak MS, Köseoğlu Y, Sertkol M, Aktaş B (2009) Cation distribution and magnetic properties of Zn doped NiFe2O4 nanoparticles synthesized by PEG-assisted hydrothermal route. J Alloys Compd 479:49–55
Kijlstra WS, Daamen JCML, Graaf JMVD, Linden BVD, Poels EK, Bliek A (1996) Inhibiting and deactivating effects of water on the selective catalytic reduction of nitric oxide with ammonia over MnOx/Al2O3. Appl Catal B Environ 7:337–357
Kim SS, Hong SC (2010) Relationship between the surface characteristics of Pt catalyst and catalytic performance on the H2-SCR. J Ind Eng Chem 16:992–996
Kim JG, Pugmire DL, Battaglia D, Langell MA (2000) Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl Surf Sci 165:70–84
Koebel M, Elsener M, Madia G (2001) Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind Eng Chem Res 40:52–59
Lee KJ, Maqbool MS, Kumar PA, Song KH, Ha HP (2013) Enhanced activity of ceria loaded Sb-V2O5/TiO2 catalysts for NO reduction with ammonia. Catal Lett 143:988–995
Leicht M, Schott FJP, Bruns M, Kureti S (2012) NOx reduction by H2 on WOx/ZrO2-supported Pd catalysts under lean conditions. Appl Catal B Environ 117–118:275–282
Li L, Zhang F, Guan N, Schreier E, Richter M (2008) NO selective reduction by hydrogen on potassium titanate supported palladium catalyst. Catal Commun 9:1827–1832
Li JH, Wang CC, Huang CJ, Sun YF, Weng WZ, Wan HL (2010a) Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene. Appl Catal A Gen 382:99–105
Li L, Wu P, Yu Q, Wu G, Guan N (2010b) Low temperature H2-SCR over platinum catalysts supported on Ti-containing MCM-41. Appl Catal B Environ 94:254–262
Li J, Chang H, Ma L, Hao J, Yang RT (2011) Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—a review. Catal Today 175:147–156
Li J, Wu G, Guan N, Li L (2012) NO selective reduction by hydrogen over bimetallic Pd–Ir/TiO2 catalyst. Catal Commun 24:38–43
Li D, Gong Y, Zhang Y, Luo C, Li W, Fu Q, Pan C (2015) Facile synthesis of carbon nanosphere/NiCo2O4 core-shell sub-microspheres for high performance supercapacitor. Sci Rep 5
Lim TH, Cho SJ, Yang HS, Engelhard MH, Kim DH (2015) Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl Catal A Gen 505:62–69
Ling F, Anthony OC, Xiong Q, Luo M, Pan X, Jia L, Huang J, Sun D, Li Q (2015): PdO/LaCoO3 heterojunction photocatalysts for highly hydrogen production from formaldehyde aqueous solution under visible light. Int J Hydrogen Energ
Liu ZQ, Xiao K, Xu QZ, Li N, Su YZ, Wang HJ, Chen S (2013) Fabrication of hierarchical flower-like super-structures consisting of porous NiCo2O4 nanosheets and their electrochemical and magnetic properties. RSC Adv 3:4372–4380
Lou XW, Deng D, Lee JY, Archer LA (2008) Thermal formation of mesoporous single-crystal Co3O4 nano-needles and their lithium storage properties. J Mater Chem 18:4397–4401
Machida M, Ikeda S, Kurogi D, Kijima T (2001) Low temperature catalytic NOx–H2 reactions over Pt/TiO2-ZrO2 in an excess oxygen. Appl Catal B Environ 35:107–116
Moni P, Kriangsak K, Sangaraju S (2014) Hierarchical nanostructured NiCo2O4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc-air batteries. Nanoscale 6:3173–3181
Olympiou GG, Efstathiou AM (2011) Industrial NOx control via H2-SCR on a novel supported-Pt nanocatalyst. Chem Eng J 170:424–432
Qi G, Yang RT, Rinaldi FC (2006) Selective catalytic reduction of nitric oxide with hydrogen over Pd-based catalysts. J Catal 237:381–392
Qing YU, Kong F, Landong LI, Guangjun WU (2010) Fast catalytic reduction of NOx by H2 over Pd-based catalysts. Chin J Catal 31:261–263
Rodríguez GCM, Saruhan B (2010) Effect of Fe/Co ratio on the phase composition of Pd-integrated perovskites and its H2-SCR of NOx performance. Appl Catal B Environ 93:304–313
Rui D, Li Q, Jia M, Wang H (2013) Facile and large-scale chemical synthesis of highly porous secondary submicron/micron-sized NiCo2O4 materials for high-performance aqueous hybrid AC-NiCo2O4 electrochemical capacitors. Electrochim Acta 107:494–502
Schott FJP, Balle P, Adler J, Kureti S (2009) Reduction of NOx by H2 on Pt/WO3/ZrO2 catalysts in oxygen-rich exhaust. Appl Catal B Environ 87:18–29
Wang Z, Dong Y, Chunyuan, Sun S (2009) Influence of temperature change on conversion characteristics of NOx in the reburning zone. Intl Conf En Env Technol 707–710
Wang X, Jiang L, Wang J, Wang R (2015a) Ag/bauxite catalysts: improved low-temperature activity and SO2 tolerance for H2-promoted NH3-SCR of NOx. Appl Catal B Environ 165:700–705
Wang X, Wen W, Mi J, Li X, Wang R (2015b) The ordered mesoporous transition metal oxides for selective catalytic reduction of NOx at low temperature. Appl Catal B Environ 176–177:454–463
Wei Y, Runduo Z, Biaohua C, Daniel D, Sébastien R (2012) New aspects on the mechanism of C3H6 selective catalytic reduction of NO in the presence of O2 over LaFe1-x(Cu, Pd)xO3-δ perovskites. Environ Sci Technol 46:11280–11288
Weirong GU, Zhou M, Wei MA, Wang Y (2012) Research progress on selective catalytic reduction de-NOx catalysts. Chem Ind Eng Progress 31:1493–1500
Xiaoling M, Bingsen Z, Yong L, Lide Y, Xuejiao W, Dang Sheng S, Wenjie S (2012) Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew Chem 51:2989–2993
Xu J, He L, Xu W, Tang H, Liu H, Han T, Zhang C, Zhang Y (2014) Facile synthesis of porous NiCo2O4 microflowers as high-performance anode materials for advanced lithium-ion batteries. Electrochim Acta 145:185–192
Xu C, Sun W, Cao L, Yang J (2015) Selective catalytic reduction of nitric oxide by hydrogen over NiFe2-xPdxO4 catalysts at low temperature. Chem Eng J 283
Yang JI, Jung H (2009) The effect of temperature on NOx reduction by H2 in the presence of excess oxygen on a Pt/Al2O3 monolithic catalyst. Chem Eng J 146:11–15
Yang S, Wang X, Chu W, Song Z, Zhao S (2011) An investigation of the surface intermediates of H2-SCR of NOx over Pt/H-FER. Appl Catal B Environ 3:380–385
Yin C, Wang L, Rivillon S, Shih AJ, Yang RT (2015) SCR of nitric oxide by hydrogen over Pd and Ir based catalysts with different supports. Catal Lett 145:1491–1499
Yu Z, Li H, Zhang X, Liu N, Tan W, Zhang X, Zhang L (2016) Facile synthesis of NiCo2O4@polyaniline core-shell nanocomposite for sensitive determination of glucose. Biosens Bioelectron 75:161–165
Yuan L, Zheng X, Duan K, Hu H, Wang J, Woo SI, Liu Z (2013) The effect of preparation conditions of Pt/Al2O3 on its catalytic performance for the H2-SCR in the presence of oxygen. Fron Env Sci Eng 7:457–463
Acknowledgments
This research is based on the work supported by the National Natural Science Foundation of China (21277045). Thanks to Zhenhua Zhou and Baosheng Tu for providing solutions to the experimental process and writing assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Highlights
The Pd-doped nickel cobaltate spinels achieved 95 % NO conversion with N2 selectivity of 100 % in the 200∼250 °C range.
SCR of NO X by hydrogen in the presence of oxygen has been significantly improved via doping Pd into NiCo2O4.
The prepared NiCo1.95Pd0.05O4 catalyst possessed better H2O and SO2 tolerance than NiCo2O4.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cai, X., Sun, W., Xu, C. et al. Highly selective catalytic reduction of NO via SO2/H2O-tolerant spinel catalysts at low temperature. Environ Sci Pollut Res 23, 18609–18620 (2016). https://doi.org/10.1007/s11356-016-7061-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7061-y