Abstract
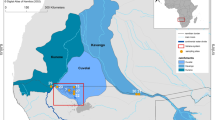
Multi-biological level assessments have become great tools to evaluate the health of aquatic ecosystems. Using this approach, a complementary study was designed to evaluate the health of yellow perch (Perca flavescens) populations in the St. Lawrence River (Quebec, Canada). In the present study, stress responses were compared at the transcriptomic, cellular, and tissue levels in yellow perch collected at six sites along the river: Lake St. François, Lake St. Louis (north and south), Beauregard Island and Lake St. Pierre (north and south). These results complement the physiological and chemical parameters as well as pathogen infection investigated in a companion paper published in the present issue. Thiobarbituric acid reactive substance (TBARS) analyses indicated the presence of oxidative stress in fish collected in the southern part of Lake St. Louis and at the downstream sites of Lake St. Pierre. High lipid peroxidation levels were found in the muscle of yellow perch caught at Beauregard Island, located downstream of the Montreal’s wastewater treatment plant, suggesting an impact of the municipal effluent on redox homeostasis. Transcriptomic results indicated the down-regulation of genes related to lipid, glucose, and retinoid in southern Lake St. Pierre as well as a decrease in retinoid storage. Overall, biochemical and molecular markers indicated that the health status of yellow perch followed a decreasing gradient from upstream to downstream of the St. Lawrence River. This gradient is representative of the cumulative negative impacts of human activities on water and habitat quality along the river.



Similar content being viewed by others
References
Alsop D, Hewitt M, Kohli M, Brown S, Van Der Kraak G (2003) Constituents within pulp mill effluent deplete retinoid stores in white sucker and bind to rainbow trout retinoic acid receptors and retinoid X receptors. Environ Toxicol Chem 22:2969–2976. doi:10.1897/02-566
Arcand-Hoy LD, Metcalfe CD (1999) Biomarkers of exposure of brown bullheads (Ameiurus nebulosus) to contaminants in the lower Great Lakes, North America. Environ Toxicol Chem 18:740–749. doi:10.1002/etc.5620180421
Barhoumi B, Clerandeau C, Gourves PY, Le Menach K, El Megdiche Y, Peluhet L, Budzinski H, Baudrimont M, Driss MR, Cachot J (2014) Pollution biomonitoring in the Bizerte lagoon (Tunisia), using combined chemical and biomarker analyses in grass goby, Zosterisessor ophiocephalus (Teleostei, Gobiidae). Mar Environ Res 101:184–195. doi:10.1016/j.marenvres.2014.07.002
Barim O, Karatepe M (2010) The effects of pollution on the vitamins A, E, C, beta-carotene contents and oxidative stress of the freshwater crayfish, Astacus leptodactylus. Ecotoxicol Environ Saf 73:138–142. doi:10.1016/j.ecoenv.2009.08.002
Batchelar KL, Kidd KA, Drevnick PE, Munkittrick KR, Burgess NM, Roberts AP, Smith JD (2013) Evidence of impaired health in yellow perch (Perca flavescens) from a biological mercury hotspot in northeastern North America. Environ Toxicol Chem 32:627–637. doi:10.1002/etc.2099
Bérubé VE, Boily MH, DeBlois C, Dassylva N, Spear PA (2005) Plasma retinoid profile in bullfrogs, Rana catesbeiana, in relation to agricultural intensity of sub-watersheds in the Yamaska River drainage basin, Quebec, Canada. Aquat Toxicol 71:109–120. doi:10.1016/j.aquatox.2004.10.018
Branchaud A, Gendron A, Fortin R, Spear PA, Anderson PD (1995) Vitamin A stores, teratogenesis, and EROD activity in white sucker, Catostomus commersoni, from Rivière des Prairies near Montréal and a reference site. Can J Fish Aquat Sci 52:1703–1713. doi:10.1139/f95-762
Brandao F, Cappello T, Raimundo J, Santos MA, Maisano M, Mauceri A, Pacheco M, Pereira P (2015) Unravelling the mechanisms of mercury hepatotoxicity in wild fish (Liza aurata) through a triad approach: bioaccumulation, metabolomic profiles and oxidative stress. Metallomics 7:1352–1363. doi:10.1039/c5mt00090d
Cajaraville MP, Cancio I, Ibabe A, Orbea A (2003) Peroxisome proliferation as a biomarker in environmental pollution assessment. Microsc Res Tech 61:191–202. doi:10.1002/jemt.10329
Carignan R (2006) The future of Lake Saint-Pierre and the St. Lawrence River as a life habitat (in French). Action Nationale 96
Chen JY, Chen JC, Wu JL (2003) Molecular cloning and functional analysis of zebrafish high-density lipoprotein-binding protein. Comp Biochem Physiol B 136:117–130. doi:10.1016/S1096-4959(03)00181-7
Christiansen HE, Mehinto AC, Yu F, Perry RW, Denslow ND, Maule AG, Mesa MG (2014) Correlation of gene expression and contaminant concentrations in wild largescale suckers: a field-based study. Sci Total Environ 484:379–389. doi:10.1016/j.scitotenv.2013.08.034
Dautremepuits C, Marcogliese DJ, Gendron AD, Fournier M (2009) Gill and head kidney antioxidant processes and innate immune system responses of yellow perch (Perca flavescens) exposed to different contaminants in the St. Lawrence River, Canada. Sci Total Environ 407:1055–1064. doi:10.1016/j.scitotenv.2008.10.004
de la Cheneliere V, Brodeur P, Mingelbier M (2014) Lake Saint-Pierre habitat restauration: a prerequisite to the yellow perch recovery (in French). Le Nate Can 138:50–61
de Lafontaine Y, Gagné F, Blaise C, Costan G, Gagnon P, Chan HM (2000) Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquat Toxicol 50:51–71. doi:10.1016/s0166-445x(99)00094-6
Defo MA, Pierron F, Spear PA, Bernatchez L, Campbell PGC, Couture P (2012) Evidence for metabolic imbalance of vitamin A2 in wild fish chronically exposed to metals. Ecotoxicol Environ Saf 85:88–95. doi:10.1016/j.ecoenv.2012.08.017
Defo MA, Spear PA, Couture P (2014) Consequences of metal exposure on retinoid metabolism in vertebrates: a review. Toxicol Lett 225:1–11. doi:10.1016/j.toxlet.2013.11.024
Defo MA, Bernatchez L, Campbell PGC, Couture P (2015) Transcriptional and biochemical markers in transplanted Perca flavescens to characterize cadmium- and copper-induced oxidative stress in the field. Aquat Toxicol 162:39–53. doi:10.1016/j.aquatox.2015.02.014
Delongchamp TM, Ridal JJ, Lean DR, Poissant L, Blais JM (2010) Mercury transport between sediments and the overlying water of the St. Lawrence River area of concern near Cornwall, Ontario. Environ Pollut 158:1487–1493. doi:10.1016/j.envpol.2009.12.030
Doyon C, Boileau S, Fortin R, Spear PA (1998) Rapid HPLC analysis of retinoids and dehydroretinoids stored in fish liver: comparison of two lake sturgeon populations. J Fish Biol 53:973–986. doi:10.1111/j.1095-8649.1998.tb00457.x
Dufour JM, Vo MN, Bhattacharya N, Okita J, Okita R, Kim KH (2003) Peroxisome proliferators disrupt retinoic acid receptor alpha signaling in the testis. Biol Reprod 68:1215–1224. doi:10.1095/biolreprod.102.010488
Gagné F, Blaise C, Andre C (2006) Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicol Environ Saf 64:329–336. doi:10.1016/j.ecoenv.2005.04.004
Gagnon C, Saulnier I (2003) Distribution and fate of metals in the dispersion plume of a major municipal effluent. Environ Pollut 124:47–55. doi:10.1016/S0269-7491(02)00433-5
Gilbert ML, Cloutier MJ, Spear PA (1995) Retinoic acid hydroxylation in rainbow trout (Oncorhynchus mykiss) and the effect of a coplanar Pcb, 3,3′,4,4′-Tetrachlorobiphenyl. Aquat Toxicol 32:177–187. doi:10.1016/0166-445X(94)00091-4
Gillis PL, Higgins SK, Jorge MB (2014) Evidence of oxidative stress in wild freshwater mussels (Lasmigona costata) exposed to urban-derived contaminants. Ecotoxicol Environ Saf 102:62–69. doi:10.1016/j.ecoenv.2013.12.026
Giraudo M, Bruneau A, Gendron A, Brodeur P, Pilote M, Marcogliese DJ, Gagnon C, Houde M (2016) Integrated spatial health assessment of yellow perch (Perca flavescens) populations from the St. Lawrence River, Quebec, Canada) part a: physiological parameters and pathogen assessment. Environ Sci Pollut Res. doi:10.1007/s11356-016-7002-9
Giuliani ME, Benedetti M, Arukwe A, Regoli F (2013) Transcriptional and catalytic responses of antioxidant and biotransformation pathways in mussels, Mytilus galloprovincialis, exposed to chemical mixtures. Aquat Toxicol 134-135:120–127. doi:10.1016/j.aquatox.2013.03.012
Goodman DS, Blaner WS (1984) Biosynthesis, absorption, and hepatic metabolism of retinol. In: Sporn MB, Roberts AB, Goodman DS (eds) The retinoids, vol 2. Academic Press, Orlando, pp. 1–39
Hontela A, Dumont P, Duclos D, Fortin R (1995) Endocrine and metabolic dysfunction in yellow perch, Perca flavescens, exposed to organic contaminants and heavy metals in the St. Lawrence river. Environ Toxicol Chem 14:725–731. doi:10.1002/etc.5620140421
Houde M, Douville M, Despatie SP, De Silva AO, Spencer C (2013) Induction of gene responses in St. Lawrence River northern pike (Esox lucius) environmentally exposed to perfluorinated compounds. Chemosphere 92:1195–1200. doi:10.1016/j.chemosphere.2013.01.099
Houde M, Berryman D, de Lafontaine Y, Verreault J (2014a) Novel brominated flame retardants and dechloranes in three fish species from the St. Lawrence River, Canada. Sci Total Environ 479-480:48–56. doi:10.1016/j.scitotenv.2014.01.105
Houde M, Giraudo M, Douville M, Bougas B, Couture P, De Silva AO, Spencer C, Lair S, Verreault J, Bernatchez L, Gagnon C (2014b) A multi-level biological approach to evaluate impacts of a major municipal effluent in wild St. Lawrence River yellow perch (Perca flavescens). Sci Total Environ 497-498:307–318. doi:10.1016/j.scitotenv.2014.07.059
Inoue D, Sei K, Ike M (2010) Disruption of retinoic acid receptor signaling by environmental pollutants. J Health Sci 56:221–230. doi:10.1248/jhs.56.221
Jackson BC, Holmes RS, Backos DS, Reigan P, Thompson DC, Vasiliou V (2013) Comparative genomics, molecular evolution and computational modeling of ALDH1B1 and ALDH2. Chem Biol Interact 202:11–21. doi:10.1016/j.cbi.2012.11.022
Kosmehl T, Otte JC, Yang L, Legradi J, Bluhm K, Zinsmeister C, Keiter SH, Reifferscheid G, Manz W, Braunbeck T, Strahle U, Hollert H (2012) A combined DNA-microarray and mechanism-specific toxicity approach with zebrafish embryos to investigate the pollution of river sediments. Reprod Toxicol 33:245–253. doi:10.1016/j.reprotox.2012.01.005
La Violette N (2004) Fluvial lakes of the Saint-Lawrence River: hydrology and human modifications (in French) Le. Nat Can 128:98–104
Lajeunesse A, Gagnon C, Gagne F, Louis S, Cejka P, Sauve S (2011) Distribution of antidepressants and their metabolites in brook trout exposed to municipal wastewaters before and after ozone treatment–evidence of biological effects. Chemosphere 83:564–571. doi:10.1016/j.chemosphere.2010.12.026
Le Cren ED (1947) The determination of the age and growth of the perch (Perea f1uviatilis) from the opercular bone. J Anim Ecol 16:188–204. doi:10.2307/1494
Liu L, Xu Y, Xu L, Wang J, Wu W, Yan Y (2015) Analysis of differentially expressed proteins in zebrafish (Danio rerio) embryos exposed to chlorpyrifos. Comp Biochem Physiol C 167:183–189. doi:10.1016/j.cbpc.2014.10.006
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Mailhot Y, Dumont P, Paradis Y, Brodeur P, Vachon N, Mingelbier M, Lecomte F, Magnan P (2015) Yellow perch (Perca flavescens) in the St. Lawrence River (Québec, Canada): population dynamics and Management in a River with contrasting pressures. In: Couture P, Pyle GG (eds) Biology of perch. CRC Press, Boca Raton
Man AK, Woo NY (2008) Upregulation of metallothionein and glucose-6-phosphate dehydrogenase expression in silver sea bream, Sparus sarba exposed to sublethal levels of cadmium. Aquat Toxicol 89:214–221. doi:10.1016/j.aquatox.2008.07.002
Marchitti SA, Brocker C, Stagos D, Vasiliou V (2008) Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol 4:697–720. doi:10.1517/17425255.4.6.697
Marcogliese DJ, Brambilla LG, Gagne F, Gendron AD (2005) Joint effects of parasitism and pollution on oxidative stress biomarkers in yellow perch Perca flavescens. Dis Aquat Org 63:77–84. doi:10.3354/dao063077
Marcogliese DJ, Dautremepuits C, Gendron AD, Fournier M (2010) Interactions between parasites and pollutants in yellow perch (Perca flavescens) in the St. Lawrence River, Canada: implications for resistance and tolerance to parasites. Can J Zool 88:247–258. doi:10.1139/z09-140
Marcogliese DJ, Blaise C, Cyr D, de Lafontaine Y, Fournier M, Gagne F, Gagnon C, Hudon C (2015) Effects of a major municipal effluent on the St. Lawrence River: a case study. Ambio 44:257–274. doi:10.1007/s13280-014-0577-9
Novák J, Beníšek M, Hilscherová K (2008) Disruption of retinoid transport, metabolism and signaling by environmental pollutants. Environ Int 34:898–913. doi:10.1016/j.envint.2007.12.024
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pelletier M (2002) Toxic contamination in sediments—Lake St-François: a century-old story. Environment Canada. Science and Technology Branch, Québec, p. 6 [En4-17/2002]
Pierron F, Bourret V, St-Cyr J, Campbell PG, Bernatchez L, Couture P (2009) Transcriptional responses to environmental metal exposure in wild yellow perch (Perca flavescens) collected in lakes with differing environmental metal concentrations (Cd, Cu, Ni). Ecotoxicology 18:620–631. doi:10.1007/s10646-009-0320-7
Pierron F, Normandeau E, Defo M, Campbell PC, Bernatchez L, Couture P (2011) Effects of chronic metal exposure on wild fish populations revealed by high-throughput cDNA sequencing. Ecotoxicology 20:1388–1399. doi:10.1007/s10646-011-0696-z
Poissant L, Beauvais C, Lafrance P, Deblois C (2008) Pesticides in fluvial wetlands catchments under intensive agricultural activities. Sci Total Environ 404:182–195. doi:10.1016/j.scitotenv.2008.05.030
Pretti C, Salvetti A, Longo V, Giorgi M, Gervasi PG (2001) Effects of beta-naphthoflavone on the cytochrome P450 system, and phase II enzymes in gilthead seabream (Sparus aurata). Comp Biochem Physiol C Toxicol Pharmacol 130:133–144
Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS (2003) Understanding the physiological role of retinol-binding protein in vitamin a metabolism using transgenic and knockout mouse models. Mol Asp Med 24:421–430. doi:10.1016/S0098-2997(03)00038-4
Richard G, Côté D, Mingelbier M, Jobin B, Morin J, Brodeur P (2011) Use of the ground in the easily flooded plain of the lake Saint-Pierre (Saint-Lawrence River) during periods 1950, 1964 and 1997: interpretation of aerial photos, digitalization and preparation of a georeferenced data basis (in French) p 42
Rodriguez-Jorquera IA, Kroll KJ, Toor GS, Denslow ND (2015) Transcriptional and physiological response of fathead minnows (Pimephales promelas) exposed to urban waters entering into wildlife protected areas. Environ Pollut 199:155–165. doi:10.1016/j.envpol.2015.01.021
Rolland RM (2000) A review of chemically-induced alterations in thyroid and vitamin a status from field studies of wildlife and fish. J Wildl Dis 36:615–635. doi:10.7589/0090-3558-36.4.615
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386. doi:10.1385/1-59259-192-2:365
Rufino-Palomares E, Reyes-Zurita FJ, Fuentes-Almagro CA, de la Higuera M, Lupianez JA, Peragon J (2011) Proteomics in the liver of gilthead sea bream (Sparus aurata) to elucidate the cellular response induced by the intake of maslinic acid. Proteomics 11:3312–3325. doi:10.1002/pmic.201000271
Sabik H, Gagnon C, Houde F, Deblois C (2004) Distribution, fate, and behavior of nonylphenol ethoxylates and degradation products in the dispersion plume of a major municipal wastewater effluent. Environ Forensic 5:61–70. doi:10.1080/15275920490454120
Shi X, Zhang S, Pang Q (2006) Vitellogenin is a novel player in defense reactions. Fish Shellfish Immunol 20:769–772. doi:10.1016/j.fsi.2005.09.005
Spear PA, Moon TW (1986) Thyroid-vitamin A interactions in chicks exposed to 3,4,3′,4′-tetrachlorobiphenyl: influence of low dietary vitamin A and iodine. Environ Res 40:188–198
Underwood BA (1984) Vitamin a in animal and human nutrition. In: Sporn MB, Roberts AB, Goodman DS (eds) The retinoids, vol 1. Academic Press, Montréal, pp. 282–392
van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Vogel S, Gamble MV, Blaner WS (1999) Biosynthesis, absorption, metabolism and transport of retinoids. Handb Exp Pharmacol 139:31–95. doi:10.1007/978-3-642-58483-1_2
Winzer K, Van Noorden CJ, Kohler A (2002) Glucose-6-phosphate dehydrogenase: the key to sex-related xenobiotic toxicity in hepatocytes of European flounder (Platichthys flesus L.)? Aquat Toxicol 56:275–288. doi:10.1016/S0166-445X(01)00215-6
Yagi K (1976) A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15:212–216. doi:10.1016/0006-2944(76)90049-1
Yutaka H, Shao-Jun D, Shuichi S, Tomonari K, Hiroshi F, Toshio T (2011) Analysis of the mechanism of skeletal deformity in fish larvae using vitamin A-induced bone deformity model. Aquaculture 351:26–33. doi:10.1016/j.aquaculture.2010.11.026
Zhang J, Xu Y, Li W, Schramm KW, Kettrup A (2002) Alterations in retinoids, tocopherol, and microsomal enzyme activities in the liver of silver carp (Hypophthalmichthys molitrix) from Ya-Er Lake, China. Bull Environ Contam Toxicol 68:660–667. doi:10.1007/s001280305
Acknowledgments
The authors wish to thank Michel Arseneau, David Sanchez, Nicolas Auclair, Rémi Bacon and local fishermen for their help during field sampling; Keven Jean and Martin Pilote for support in the laboratory and François Boudreault for the map. This study was funded by Environment Canada’s Strategic Technology Application of Genomics in the Environment (STAGE) programme, supports from the St. Lawrence Action Plan and the Ministère des Forêts, de la Faune et des Parcs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Thomas Braunbeck
Electronic supplementary material
ESM 1
(DOCX 551 kb)
Rights and permissions
About this article
Cite this article
Bruneau, A., Landry, C., Giraudo, M. et al. Integrated spatial health assessment of yellow perch (Perca flavescens) populations from the St. Lawrence River (QC, Canada), part B: cellular and transcriptomic effects. Environ Sci Pollut Res 23, 18211–18221 (2016). https://doi.org/10.1007/s11356-016-7001-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7001-x