Abstract
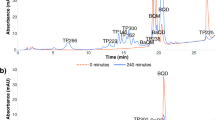
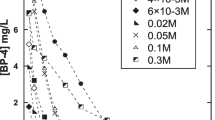
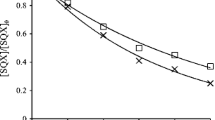
This study investigated the reaction kinetics and the transformation by-products of acebutolol during aqueous chlorination. Acebutolol is one of the commonly used β-blockers for the treatment of cardiovascular diseases. It has been frequently detected in the aquatic environment. In the kinetics study, the second-order rate constant for the reaction between acebutolol and chlorine (k app) was determined at 25 ± 0.1 °C. The degradation of acebutolol by free available chlorine was highly pH dependence. When the pH increased from 6 to 8, it was found that the k app for the reaction between acebutolol and free available chlorine was increased from 1.68 to 11.2 M−1 min−1. By comparing with the reported k app values, the reactivity of acebutolol toward free available chlorine was found to be higher than atenolol and metoprolol but lower than nadolol and propranolol. Characterization of the transformation by-products formed during the chlorination of acebutolol was carried out using liquid chromatography-quadrupole time-of-flight high-resolution mass spectrometry. Seven major transformation by-products were identified. These transformation by-products were mainly formed through dealkylation, hydroxylation, chlorination, and oxidation reactions.




Similar content being viewed by others
References
Abia L, Armesto XL, Canle ML, Garcia MV, Santaballa JA (1998) Oxidation of aliphatic amines by aqueous chlorine. Tetrahedron 54:521–530
Acero JL, Benitez FJ, Real FJ, Roldan G (2010) Kinetics of aqueous chlorination of some pharmaceuticals and their elimination from water matrices. Water Res 44:4158–4170
Adam LC, Gordon G (1995) Direct and sequential potentiometric determination of hypochlorite, chlorite, and chlorate ions when hypochlorite ion is present in large excess. Anal Chem 67:535–540
Babic S, Horvat AJM, Pavlovic DM, Macan MK (2007) Determination of pKa values of active pharmaceutical ingredients. Trends Anal Chem 26:1043–1061
Bedner M, MacCrehan WA (2006) Reactions of the amine-containing drugs fluoxetine and metoprolol during chlorination and dechlorination processes used in wastewater treatment. Chemosphere 65:2130–2137
Cai MQ, Feng L, Jiang J, Qi F, Zhang LQ (2013) Reaction kinetics and transformation of antipyrine chlorination with free chlorine. Water Res 47:2830–2842
Chamberlain E, Adams C (2006) Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine. Water Res 40:2517–2526
Daneshvar A, Svanfelt J, Kronberg L, Prѐvost M, Weyhenmeyer GA (2010) Seasonal variations in the occurrence and fate of basic and neutral pharmaceuticals in a Swedish river-lake system. Chemosphere 80:301–309
Deborde M, Gunten UV (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanism: a critical review. Water Res 42:13–51
Dodd MC, Shah AD, Gunten UV, Huang CH (2005) Interactions of fluoroquinolone antibacterial agents with aqueous chlorine: reaction kinetics, mechanism, and transformation pathways. Environ Sci Technol 39:7065–7076
El Najjar NM, Deboede M, Journel R, Vel Leitner NK (2013) Aqueous chlorination of levofloxacin: kinetics and mechanistic study, transformation product identification and toxicity. Water Res 47:121–129
Gabet-Giraud V, Miege C, Choubert JM, Ruel SM, Coquery M (2010) Occurrence and removal of estrogens and beta blockers by various wastewater treatment plants. Sci Total Environ 408:4257–4269
Gabet-Giraud V, Miege Jacquet R, Coquery M (2014) Impact of wastewater treatment plants on receiving surface waters and a tentative risk evaluation: the case of estrogens and beta blockers. Environ Sci Pollut Res 21:1708–1722
Gallard H, Gunten UV (2002) Chlorination of phenols: kinetics and formation of chloroform. Environ Sci Technol 36:884–890
Garcia SAO, Pinto GP, Encina PAG, Mata RI (2014) Ecotoxicity ad environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants. Ecotoxicology 23:1517–1533
Ge F, Zhu L, Wang J (2008) Distribution of chlorination products of phenols under various pHs in water disinfection. Desalination 225:156–166
Glassmeyer ST, Shoemaker JA (2005) Effects of chlorination on the persistence of pharmaceuticals in the environment. Bull Environ Contam Toxicol 74:24–31
Kibbey TCG, Paruchuri R, Sabatini DA, Chen L (2007) Adsorption of beta blockers to environmental surfaces. Environ Sci Technol 41:5349–5356
Kim SD, Cho J, Kim IS, Vanderford BJ, Synder SA (2007) Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res 41:1013–1021
Kosma CI, Lambropoulou DA, Albanis TA (2010) Occurrence ad removal of PPCPs in municipal and hospital wastewaters in Greece. J Hazard Mater 179:804–817
Lahti M, Oikari A (2011) Pharmaceuticals in settleable particulate material in urban and non-urban waters. Chemosphere 85:826–831
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641
Osachoff HL, Mohammadali M, Skirrow RC, Hall ER, Brown LYL, Aggelen GCV, Kennedy CJ, Helbing CC (2014) Evaluating the treatment of a synthetic wastewater containing a pharmaceutical and personal care product chemical cocktail: compound removal efficiency and effects on juvenile rainbow trout. Water Res 62:271–280
Padhye LP, Yao H, Kung’u FT, Huang C (2014) Year-long evaluation on the occurrence and fate of personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res 51:266–276
Pinkston KE, Sedlak DL (2004) Transformation of aromatic ether and amine-containing pharmaceuticals during chlorine disinfection. Environ Sci Technol 38:4019–4025
Quintana JB, Rodil R, Cela R (2012) Reaction of β-blockers and β-agonist pharmaceuticals with aqueous chlorine. Investigation of kinetics and by-products by liquid chromatography quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem 403:2385–2395
Salem AA, Wasfi IA, Al-Nassibi SS (2012) Trace determination of β-blockers and β2-agonists in distilled and waste-waters using liquid chromatography-tandem mass spectrometry and solid-phase extraction. J Chromatogr B 908:27–38
Sharma VK (2008) Oxidative transformations of environmental pharmaceuticals by Cl2, CIO2, O3, and Fe(VI): kinetics assessment. Chemosphere 73:1379–1386
Soufan M, Deborde M, Delmont A, Legube B (2013) Aqueous chlorination of carbamazepine: kinetic study and transformation product identification. Water Res 47:5076–5087
Sun Q, Lv M, Hu A, Yang X, Yu CP (2014) Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J Hazard Mater 277:69–75
Szabo J, Minamyer S (2014) Decontamination of chemical agents from drinking water infrastructure: a literature review and summary. Environ Int 72:119–123
Tay KS, Madehi N (2014) Ozonation of acebutolol in aqueous solution: ozonation by-products and degradation pathway. Sep Purif Technol 135:48–63
Tay KS, Rahman NA, Abas MRB (2010) Ozonation of parabens in aqueous solution: kinetics and mechanism of degradation. Chemosphere 81:1446–1453
Tian F, Liu W, Guo G, Qiang Z, Zhang C (2014) Kinetics and mechanism of dimethoate chlorination during drinking water treatment. Chemosphere 103:181–187
Zarelli A, DellaGreca M, Parollisi A, Iesce MR, Cermola F, Temussi F, Isidori M, Lavorgna M, Passananti M, Previtera L (2012) Chemical fate and genotoxic risk associated with hypochlorite treatment of nicotine. Sci Total Environ 426:132–138
Zhang TY, Xu B, Hu CY, Li M, Xia SJ, Tian FX, Gao NY (2013) Degradation kinetics and chloropicrin formation during aqueous chlorination of dinoseb. Chemosphere 93:2662–2668
Zhang Y, Zhuang Y, Geng J, Ren H, Zhang Y, Ding L, Xu K (2015) Inactivation of antibiotic resistance genes in municipal wastewater effluent by chlorination and sequential UV/chlorination disinfection. Sci Total Environ 512–513:125–132
Acknowledgments
This research was supported financially by the Fundamental Research Grant Scheme (FP043-2013A) of Ministry of Higher Education Malaysia, and University of Malaya (PPP PG075-2014B and UMRG RG342-15-AFR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 147 kb)
Rights and permissions
About this article
Cite this article
Khalit, W.N.A.W., Tay, K.S. Aqueous chlorination of acebutolol: kinetics, transformation by-products, and mechanism. Environ Sci Pollut Res 23, 2521–2529 (2016). https://doi.org/10.1007/s11356-015-5470-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5470-y