Abstract
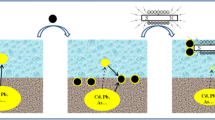
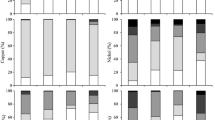
Nano-zero-valent iron/activated carbon (nZVI/AC) composite was evaluated for its effectiveness in the stabilization of Cu, Pb, Cd, and Cr in dredged river sediment. Synthetic precipitation leaching procedure (SPLP) and toxicity characteristic leaching procedure (TCLP) were adopted to compare the effects of nZVI/AC dosage, particle size, time duration, and temperature on heavy metal leachability. The results show that leachability dropped considerably with the addition of nZVI/AC and powdered particles in the size of 0.075–0.18 mm was more effective in stabilization than granular ones. Stabilization effect was stable in long-term and robust against changes in temperature. Tessier sequential extraction revealed that heavy metals were associated with solid particle, inorganic or organic matters in sediment. The addition of nZVI/AC was able to convert relatively weakly bound heavy metals into more strongly bound species and thus reduce the bioavailability and toxicity. Also, the standard potential of heavy metals may decide the mechanism of stabilization process.






Similar content being viewed by others
References
Ahn S, Werner D, Karapanagioti HK, McGlothlin DR, Zare RN, Luthy RG (2005) Phenanthrene and pyrene sorption and intraparticle diffusion in polyoxymethylene, coke, and activated carbon. Environ Sci Technol 39(17):6516–6526
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86(1):24–36
Anju M, Banerjee DK (2010) Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 78(11):1393–1402
Chen WF, Pan L, Chen LF, Wang Q, Yan CC (2014) Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: iron loading, kinetics and pathway. RSC Adv 4(87):46689–46696
Cho YM, Smithenry DW, Ghosh U, Kennedy AJ, Millward RN, Bridges TS, Luthy RG (2007) Field methods for amending marine sediment with activated carbon and assessing treatment effectiveness. Mar Environ Res 64(5):541–555
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–125
Dhanakumar S, Solara G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113(3):145–151
Fernández-Cadena JC, Andrade S, Silva-Coello CL, De la Iglesia R (2014) Heavy metal concentration in mangrove surface sediments from the north-west coast of South America. Mar Pollut Bull 82(1–2):221–226
Huang CC, Lo SL, Lien HL (2012) Zero-valent copper nanoparticles for effective dechlorination of dichloromethane using sodium borohydride as a reductant. Chem Eng J 203:95–100
Idris AM, Eltayeb MAH, Potgieter-Vermaak SS, Van Grieken R, Potgieter JH (2007) Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem J 87(2):104–112
Ke Y, Chai LY, Min XB, Tang CJ, Chen J, Wang Y, Liang YJ (2014) Sulfidation of heavy-metal-containing neutralization sludge using zinc leaching residue as the sulfur source for metal recovery and stabilization. Miner Eng 61(6):105–112
Kersten M, Förstner U (1986) Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Sci Technol 18:121–130
Lebo JA, Huckins JN, Petty JD, Crranor WL, Ho KT (2003) Comparisons of coarse and fine versions of two carbons for reducing the bioavailabilities of sediment-bound hydrophobic organic contaminants. Chemosphere 50(10):1309–1317
Lee JY, Hozalski RM, Amlod WA (2007) Effects of dissolved oxygen and iron aging on the reduction of trichloronitromethane, trichloracetonitrile, and trichloropropanone. Chemosphere 66(11):2127–2135
Lee SB, An JS, Kim YJ, Nam K (2011) Binding strength-associated toxicity reduction by birnessite and hydroxyapatite in Pb and Cd contaminated sediments. J Hazard Mater 186:2117–2122
Li XQ, Zhang WX (2007) Sequestration of metal cations with zero valent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Lu Q, Sorial GA (2004) Adsorption of phenolics on activated carbon-impact of pore size and molecular oxygen. Chemosphere 55(5):671–679
Lv XS, Xu J, Jiang GM, Xu XH (2011) Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 85:1204–1209
Lv XS, Xu J, Jiang GM, Tang J, Xu X (2012) Highly active nanoscale zero-valent iron (nZVI)-Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J Colloid Interf Sci 369:460–469
Magalhaes F, Pereira MC, Fabris JD, Bottrel SEC, Sansiviero MTC, Amaya A, Tancredi N, Lago RM (2009) Novel highly reactive and regenerable carbon/iron composites prepared from tar and hematite for the reduction of Cr(VI) contaminant. J Hazard Mater 165:1016–1022
Mamindy-Pajany Y, Hurel C, Geret F, Romeo M, Marmier N (2013) Comparison of mineral-based amendments for ex-situ stabilization of trace elements (As, Cd, Cu, Mo, Ni, Zn) in marine dredged sediments: a pilot-scale experiment. J Hazard Mater 252–253(5):213–219
Masciandaro G, Di Biase A, Macci C, Peruzzi E, Iannelli R, Doni S (2014) Phytoremediation of dredged marine sediment: monitoring of chemical and biochemical processes contributing to sediment reclamation. J Environ Manag 134(2):166–174
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85(1–2):145–163
Nemati K, Bakar NKA, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192(1):402–410
Pardo R, Vega M, Debán L, Cazurro C, Carretero C (2008) Modelling of chemical fractionation patterns of metals in soils by two-way and three-way principal component analysis. Anal Chim Acta 606(1):26–36
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2–3):633–640
Qian G, Chen W, Lim TT, Chui P (2009) In-situ stabilization of Pb, Zn, Cu, Cd and Ni in the multi-contaminated sediments with ferrihydrite and apatite composite additives. J Hazard Mater 170(2–3):1093–1100
Qiu X, Fang Z, Liang B, Gu F, Xu Z (2011) Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J Hazard Mater 193:70–81
Rakowska MI, Kupryianchyk D, Koelmans AA, Grotenhuis T, Rijnaarts HH (2014) Equilibrium and kinetic modeling of contaminant immobilization by activated carbon amended to sediments in the field. Water Res 67(12):96–104
Shafie NA, Aris AZ, Haris H (2014) Geoaccumulation and distribution of heavy metals in the urban river sediment. Int J Sediment Res 29(3):368–377
Sun H, Zhou G, Liu S, Ang HM, Tadé MO, Wang S (2012) Nano-Fe0 encapsulated in microcarbon spheres: synthesis, characterization, and environmental applications. ACS Appl Mater Interf 4:6235–6241
Sutherland RA (1998) Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia 389:153–167
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tsang DCW, Lo IMC (2006) Competitive Cu and Cd sorption and transport in soils: a combined batch kinetics, column, and sequential extraction study. Environ Sci Technol 40(21):6655–6661
Tseng HH, Su JG, Liang C (2011) Synthesis of granular activated carbon/zero valent iron composites for simultaneous adsorption/dechlorination of trichloroethylene. J Hazard Mater 192(2):500–506
USEPA (1992) Toxicity characteristic leaching procedure (TCLP), SW-846 Method 1311. Federal Register, 55 (March 29), Washington, DC
Wang F, Yao J, Si Y, Chen H, Russel M, Chen K, Qian YG, Zaray G, Bramanti E (2010) Short-time effect of heavy metals upon microbial community activity. J Hazard Mater 173:510–516
Xu G, Liu M, Li G (2013) Stabilization of heavy metals in lightweight aggregate made from sewage sludge and river sediment. J Hazard Mater 260(1–3):74–81
Yan DYS, Tang IY, Lo IMC (2014) Development of controlled low-strength material derived from beneficial reuse of bottom ash and sediment for green construction. Constr Build Mater 64(8):201–207
Zhou X, Guo J, Lin K, Huang K, Deng J (2013) Leaching characteristics of heavy metals and brominated flame retardants from waste printed circuit boards. J Hazard Mater 246(2):96–102
Acknowledgments
This work was supported by the Shanghai Natural Science Foundation (14ZR1428900), the National Natural Science Foundation of China (51078233), the Returned Overseas Chinese Scholars, the State Education Ministry (SEM2013), and the Shanghai Committee of Science and Technology (13230502300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Chen, Wf., Zhang, J., Zhang, X. et al. Investigation of heavy metal (Cu, Pb, Cd, and Cr) stabilization in river sediment by nano-zero-valent iron/activated carbon composite. Environ Sci Pollut Res 23, 1460–1470 (2016). https://doi.org/10.1007/s11356-015-5387-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5387-5