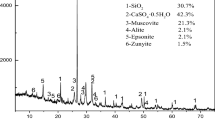
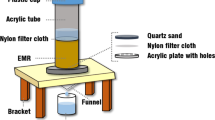
Abstract
Electrolytic manganese solid waste (EMSW) is a by-product of electrolytic manganese production and generally contains a high concentration of soluble Mn(II) (2000–3000 mg/L). Millions of tons of EMSW are stored in China, and the environmental pollution caused by manganese in this waste product is concerning. Unfortunately, little attention has been paid to the immobilization of manganese from industrial solid waste because manganese is absent from toxicological identification standards, and there is a lack of relevant quality standards in China. The objectives of this study were to immobilize soluble Mn(II) using chemical reagents, to analyze the immobilization mechanism, and to identify the most economical reagents. We investigated the immobilization degrees of soluble Mn(II) achieved by the reagents quicklime (CaO), carbonates (NaHCO3 and Na2CO3), phosphates (Na3PO4, Na2HPO4, NH4H2PO4, and Ca10(PO4)6(OH)2), and caustic magnesia (MgO) both individually and in combination. Our results showed that the use of 9 % CaO+ 5 % NaHCO3, 9 % CaO+ 5 % Na3PO4, 10 % MgO alone, or with 1–5 % NaHCO3 or 1–5 % Na2CO3 can reduce the amount of Mn(II) leached to 100 mg/kg when the eluate pH was in the range of 6–9. The most economical reagent treatments were determined using K-means cluster analysis. Analysis of the immobilization mechanism showed that CaO + NaHCO3 may be favorable for immobilizing soluble Mn(II) as precipitation and oxidation products because the addition of NaHCO3 releases OH− and buffers the system.







Similar content being viewed by others
References
Bhattacharyya KG, Gupta SS (2007) Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: influence of acid activation. J Colloid Interface Sci 310:411–424
Bone B, Barnard L, Boardman D, Carey P, Hills C, Jones H, MacLeod C, Tyrer M (2004) Review of scientific literature on the use of stabilisation/solidification for the treatment of contaminated soil, solid waste and sludges, Bristol, UK
Bricker OP (1965) Some stability relations in system Mn-O2-H2O at 25 degrees and 1 atmosphere total pressure. Am Mineral 50:1296–1354
Catalan LJ, Yin G (2003) Comparison of calcite to quicklime for amending partially oxidized sulfidic mine tailings before flooding. Environ Sci Technol 37:1408–1413
Chen Q, Ke Y, Zhang L, Tyrer M, Hills CD, Xue G (2009) Application of accelerated carbonation with a combination of Na2CO3 and CO2 in cement-based solidification/stabilization of heavy metal-bearing sediment. J Hazard Mater 166:421–427
Conner JR, Hoeffner SL (1998) A critical review of stabilization/solidification technology. Crit Rev Environ Sci Technol 28:397–462
Cortina J-L, Lagreca I, De Pablo J, Cama J, Ayora C (2003) Passive in situ remediation of metal-polluted water with caustic magnesia: evidence from column experiments. Environ Sci Technol 37:1971–1977
Cubukcuoglu B, Ouki S (2012) Solidification/stabilisation of electric arc furnace waste using low grade MgO. Chemosphere 86:789–796
Dash SK, Hussain M (2012) Lime stabilization of soils: reappraisal. J Mater Civ Eng 24:707–714
Dell’Orso M, Mangialardi T, Paolini AE, Piga L (2012) Evaluation of the leachability of heavy metals from cement-based materials. J Hazard Mater 227-228:1–8
Du B, Zhou C, Dan Z, Luan Z, Duan N (2014) Preparation and characteristics of steam-autoclaved bricks produced from electrolytic manganese solid waste. Constr Build Mater 50:291–299
Duan N, Wang F, Zhou CB, Zhu C, Yu H (2010) Analysis of pollution materials generated from electrolytic manganese industries in China. Resour Conserv Recycl 54:506–511
Dutré V, Vandecasteele C (1998) Immobilization mechanism of arsenic in waste solidified using cement and lime. Environ Sci Technol 32:2782–2787
Erikson KM, Aschner M (2003) Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int 43:475–480
Fernandez Bertos M, Simons S, Hills C, Carey P (2004) A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J Hazard Mater 112:193–205
Gerber G, Leonard A, Hantson P (2002) Carcinogenicity, mutagenicity and teratogenicity of manganese compounds. Crit Rev Oncol Hematol 42:25–34
Guo G, Zhou Q, Ma LQ (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Hills C, Sweeney R, Buenfeld N (1999) Microstructural study of carbonated cement-solidified synthetic heavy metal waste. Waste Manag 19:325–331
Ikhsan J, Johnson BB, Wells JD (1999) A comparative study of the adsorption of transition metals on kaolinite. J Colloid Interface Sci 217:403–410
Johannesson B, Utgenannt P (2001) Microstructural changes caused by carbonation of cement mortar. Cem Concr Res 31:925–931
Kessick MA, Morgan JJ (1975) Mechanism of autoxidation of manganese in aqueous solution. Environ Sci Technol 9:157–159
Kogbara RB, Al-Tabbaa A (2011) Mechanical and leaching behaviour of slag-cement and lime-activated slag stabilised/solidified contaminated soil. Sci Total Environ 409:2325–2335
Lata H, Garg VK, Gupta RK (2008) Sequestration of nickel from aqueous solution onto activated carbon prepared from Parthenium hysterophorus. J Hazard Mater 157:503–509
Li M, Luo Y, Su Z (2007) Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ Pollut 147:168–175
Luo L (2012) Environmental risk research of selenium and heavy metal pollutants in industry of electrolytic manganese. Anhui University of Science and Technology, Huainan
Ma QY, Logan TJ, Traina SJ, Ryan JA (1994) Effects of NO3 −, Cl−, F−, SO4 2−, and CO3 2−on Pb2+ immobilization by hydroxyapatite. Environ Sci Technol 28:408–418
Malviya R, Chaudhary R (2004) Study of the treatment effectiveness of a solidification/stabilization process for waste bearing heavy metals. J Mater Cycles Waste Manage 6:147–152
Mavropoulos E, Rossi AM, Costa AM, Perez CAC, Moreira JC, Saldanha M (2002) Studies on the mechanisms of lead immobilization by hydroxyapatite. Environ Sci Technol 36:1625–1629
Morgan JJ (1967) Chemical equilibria and kinetic properties of manganese in natural waters. Principles and applications of water chemistry. Faust SD, Hunter JV (ed). 561–624 pp
Morgan JJ (2005) Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim Cosmochim Acta 69:35–48
Olgun A, Atar N (2011) Removal of copper and cobalt from aqueous solution onto waste containing boron impurity. Chem Eng J 167:140–147
Qiu W, Zheng Y (2009) Removal of lead, copper, nickel, cobalt, and zinc from water by a cancrinite-type zeolite synthesized from fly ash. Chem Eng J 145:483–488
Quina MJ, Bordado J, Quinta-Ferreira RM (2010) Chemical stabilization of air pollution control residues from municipal solid waste incineration. J Hazard Mater 179:382–392
Rocha SD, Mansur MB, Ciminelli VS (2004) Kinetics and mechanistic analysis of caustic magnesia hydration. J Chem Technol Biotechnol 79:816–821
Rodríguez-Jordá M, Garrido F, García-González MT (2010) Assessment of the use of industrial by-products for induced reduction of As, and Se potential leachability in an acid soil. J Hazard Mater 175:328–335
Rötting TS, Ayora C, Carrera J (2008) Improved passive treatment of high Zn and Mn concentrations using caustic magnesia (MgO): particle size effects. Environ Sci Technol 42:9370–9377
Seaman JC, Arey JS, Bertsch PM (2001) Immobilization of nickel and other metals in contaminated sediments by hydroxyapatite addition. J Environ Qual 30:460–469
State Environmental Protection Administration of China (2007) Identification standards for hazardous wastes-identification for extraction toxicity. GB 5085. 3–2007
Standard for pollution control on the storage and disposal site for general industrial solid wastes, Ministry of Environmental Protection of the People’s Republic of China. GB 18599–2001
Valls S, Vazquez E (2001) Accelerated carbonatation of sewage sludge–cement–sand mortars and its environmental impact. Cem Concr Res 31:1271–1276
Vartanian J-P, Sala M, Henry M, Wain-Hobson S, Meyerhans A (1999) Manganese cations increase the mutation rate of human immunodeficiency virus type 1 ex vivo. J Gen Virol 80:1983–1986
Wang Y (2012) Human heath risk study of selenium and heavy metals pollution in electrolysis manganese industry. Anhui University of Science and Technology, Huainan
Wang Z, Xiong W, Tebo BM, Giammar DE (2014) Oxidative UO2 dissolution induced by soluble Mn (III). Environ Sci Technol 48:289–298
Williford C, Chen W-Y, Shamas NK, Wang LK (2007) Lime stabilization. Biosolids Treat Process 6:207–241
Yola ML, Eren T, Atar N (2014a) A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem Eng J 250:288–294
Yola ML, Eren T, Atar N, Wang S (2014b) Adsorptive and photocatalytic removal of reactive dyes by silver nanoparticle-colemanite ore waste. Chem Eng J 242:333–340
Zhou C, Du B, Wang N, Chen Z (2014) Preparation and strength property of autoclaved bricks from electrolytic manganese residue. J Clean Prod 84:707–714
Acknowledgments
We thank three anonymous reviewers whose comments improved the manuscript. We would like to gratefully acknowledge the National Key Project of Scientific and Technical Supporting Programs by the Ministry of Science & Technology of China (Grant No. 2012BAF03B03) and the fundamental research funds for central public welfare research institutes (2013-YSKY-20).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 34 kb)
Rights and permissions
About this article
Cite this article
Du, B., Hou, D., Duan, N. et al. Immobilization of high concentrations of soluble Mn(II) from electrolytic manganese solid waste using inorganic chemicals. Environ Sci Pollut Res 22, 7782–7793 (2015). https://doi.org/10.1007/s11356-015-4197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4197-0