Abstract
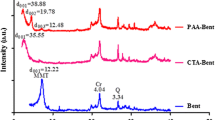
Bentonite clay was modified using quaternary ammonium cations, viz. phenyltrimethylammonium (PTMA), hexadecyltrimethylammonium (HDTMA), trioctylmethylammonium (TOMA) [100 % of cation exchange capacity of clay], and stearylkonium (SK) [100 % (SK-I) and 250 % (SK-II) of cation exchange capacity of clay]. The organoclays were characterized using X-ray diffraction (XRD), infrared (IR) spectroscopy, and scanning electron microscopy (SEM). Atrazine adsorption on modified clays was studied using a batch method. Bentonite clay was a poor adsorbent of atrazine as 9.4 % adsorption was observed at 1 μg mL−1 atrazine concentration. Modification of clay by PTMA cation did not improve atrazine adsorption capacity. However, atrazine adsorption in HDTMA-, TOMA-, and SK-bentonites varied between 49 and 72.4 % and data fitted well to the Freundlich adsorption isotherm (R > 0.96). Adsorption of atrazine in organoclays was nonlinear and slope (1/n) values were <1. The product of Freundlich adsorption constants, K f(1/n) in HDTMA-, TOMA-, and SK-I-bentonites was 239.2, 302.4, and 256.6, respectively, while increasing the SK cation loading in the clay (SK-II) decreased atrazine adsorption [K f(1/n) − 196.4]. Desorption of atrazine from organoclays showed hysteresis and TOMA- and SK-I-bentonites were the best organoclays to retain the adsorbed atrazine. Organoclays showed better atrazine removal from wastewater than an aqueous solution. The synthesized organoclays may find application in soil and water decontamination and as a carrier for atrazine-controlled released formulations.






Similar content being viewed by others
References
Abate G, Masini JC (2005) Sorption of atrazine, propazine, deethylatrazine, deisopropylatrazine and hydroxyatrazine on organovermiculite. J Braz Chem Soc 16:936–943
Akçay G, Yurdakoç MK (1999) Nonyl- and dodecylamines intercalated bentonite and illite from Turkey. Turk J Chem 23:105–113
Borisover M, Graber ER, Bercovich F, Gerstl Z (2001) Suitability of dye-clay complexes for removal of non-ionic organic compounds from aqueous solution. Chemosphere 44:1033–1040
Boyd SA, Jaynes WF, Ross BS (1991) Immobilization of organic contaminants by organo-clays: application to soil restoration and hazardous waste contaminants. In: Baker RA (ed) Organic substances and sediments in water, vol 1, CRC Press. Boca Raton, FL, pp 181–200
Celis R, Hermosín MC, Carrizosa MJ, Cornejo J (2002) Inorganic and organic clays as carriers for controlled release of herbicide hexazinone. J Agril Food Chem 50:2324–2330
El-Nahhal Y, Nir S, Polubesova T, Margulies L, Rubin B (1998) Movement of metolachlor in soil: effect of new organoclay formulations. Pest Sci 55:857–864
El-Nahhal Y, Nir S, Polubesova T, Margulies L, Rubin B (1999) Leaching, phytotoxicity and weed control of new formulations of alachlor. J Agril Food Chem 42:1223–1227
El-Nahhal Y, Nir S, Serban C, Rabinovitch O, Rubin B (2000) Montmorillonite-phenyltrimethylammonium yields environmentally improved formulations of hydrophobic herbicides. J Agril Food Chem 48:4791–4801
Farmer VC (1974) The layer silicates. In: Farmer VC (ed) The infrared spectra of minerals, Mineralogical Society Monograph 4. London, pp 331–363
Gaynor JD, Mactavish DC, Findlay WI (1995) Atrazine and metolachlor loss in surface and subsurface runoff from three tillage treatments in corn. J Environ Qual 24:246–256
Giles CH, McEvans TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI: a system of classification of adsorption isotherms and its use in diagnosis of desorption mechanism and measurement of specific surface areas of solids. J Chem Soc 3:3973–3993
Gilliom RJ (2007) Pesticides in U.S. streams and groundwater. Environ Sci Technol 41:3408–3414
Groisman L, Rav-Acha C, Gerstl Z, Mingelgrin U (2004) Sorption of organic compounds of varying hydrophobicities from water and industrial wastewater by long- and short-chain organoclays. Appl Clay Sci 24:159–166
Hermosín MC, Calderon MJ, Aguer JP, Cornejo J (2001) Organoclays for controlled release of the herbicide fenuron. Pest Manag Sci 57:803–809
Jackson ML (1967) Soil chemical analysis. Prentice Hall Inc., New Delhi, India
Keeney DR, Nelson DW (1989) Nitrogen inorganic forms. In: Page AL (ed) Methods of soil analysis, part 2: chemical and microbiological properties. Soil Science Society of America and American Society of Agronomy, Madison, WI, pp 643–698
Li Z, Alessi D, Allen L (2002) Influence of quaternary ammonium on sorption of selected metal cations onto clinoptilolite zeolite. J Environ Qual 31:1106–1114
Mishael YG, Undabeytia T, Rabinovitz O, Rubin B, Nir S (2002) Slow-release formulations of sulfometuron incorporated in micelles adsorbed on montmorillonite. J Agril Food Chem 50:2864–2869
Poinke HB, Glotfelty DE, Lucas AD, Urban JB (1998) Pesticide contamination of groundwater in the Mahantango Creek Watershed. J Environ Qual 7:76–84
Sánchez-Camazano M, Sánchez-Martín MJ (1994) Organo-clays as adsorbents for azinphosmethyl and dichlorvos in aqueous medium. Water Air Soil Pollut 74:19–28
Sánchez-Martín MJ, Rodriguez-Cruz MS, Andrades MS, Sánchez-Camazano M (2006) Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: influence of clay type and pesticide hydrophobicity. Appl Clay Sci 31:216–228
Trigo C, Koskinen WC, Celis R, Sadowsky MJ, Hermosín MC, Cornejo J (2010) Bioavailability of organoclay formulations of atrazine in soils. J Agril Food Chem 58:11857–11863
Undabeytia T, Nir S, Rubin B (2000) Organo-clay formulation of the hydrophobic herbicide norflurazon yield reduced leaching. J Agril Food Chem 48:4767–4773
Undabeytia T, Nir S, Sánchez-Verdejo T, Villaverde J, Maqueda C, Morillo E (2008) A clay-vesicle system for water purification from organic pollutants. Water Res 42:1211–1219
USDHHS, United States Department of Health and Human Services (2003) Toxicological profile for atrazine. Public Health Service, Agency for Toxic Substances and Disease Registry (ATDSR), Atlanta, GA. http://www.atsdr.cdc.gov/ToxProfiles/tp153.pdf. Accessed 21 March 2014
USEPA, United States Environment Protection Agency (2009) National primary drinking water regulations. EPA 816-F-09-004, May 2009. http://www.epa.gov/safewater/consumer/pdf/mcl.pdf. Accessed 21 March 2014
USEPA, United States Environmental Protection Agency (2006) Decision documents for atrazine. Washington D.C., USA. http://www.epa.gov/oppsrrd1/REDs/atrazine_combined_docs.pdf. Accessed 7 February 2013
Wang Q, Yang W, Liu W (1999) Adsorption of acetanilide herbicides on soils and its correlation with soil properties. Pestic Sci 55:1103–1108
Zhang ZZ, Sparks DL, Scrivner SC (1993) Sorption and desorption of quaternary amine cations on clays. Environ Sci Technol 27:1625–1631
Acknowledgments
Anirban Dutta was supported by INSPIRE Fellowship from the Department of Science and Technology (DST), Government of India, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dutta, A., Singh, N. Surfactant-modified bentonite clays: preparation, characterization, and atrazine removal. Environ Sci Pollut Res 22, 3876–3885 (2015). https://doi.org/10.1007/s11356-014-3656-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3656-3