Abstract
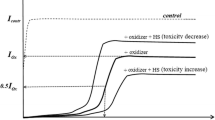
The paper considers mechanisms of detoxification of pollutant solutions by water-soluble humic substances (HSs), natural detoxifying agents. The problems and perspectives of bioassay application for toxicity monitoring of complex solutions are discussed from ecological point of view. Bioluminescence assays based on marine bacteria and their enzymes are of special attention here; they were shown to be convenient tools to study the detoxifying effects on cellular and biochemical levels. The advantages of bioluminescent enzymatic assay for monitoring both integral and oxidative toxicities in complex solutions of model pollutants and HS were demonstrated. The efficiencies of detoxification of the solutions of organic oxidizers and salts of metals (including radioactive ones) by HS were analyzed. The dependencies of detoxification efficiency on time of exposure to HS and HS concentrations were demonstrated. Antioxidant properties of HS were considered in detail. The detoxifying effects of HS were shown to be complex and regarded as ‘external’ (binding and redox processes in solutions outside the organisms) and/or ‘internal’ organismal processes. The paper demonstrates that the HS can stimulate a protective response of bacterial cells as a result of (1) changes of rates of biochemical reactions and (2) stabilization of mucous layers outside the cell walls. Acceleration of auto-oxidation of NADH, endogenous reducer, by HS was suggested as a reason for toxicity increase in the presence of HS due to abatement of reduction ability of intracellular media.








Similar content being viewed by others
References
Agrawal A, Kumari S, Sahu KK (2009) Iron and copper recovery/removal from industrial wastes: a review. Ind Eng Chem Res 48:6145–6161
Al-Abri M, Dakheel A, Tizaoui C, Hilal N (2010) Combined humic substance and heavy metals coagulation, and membrane filtration under saline conditions. Desalination 253:46–50
Alexandrova M, Rozhko T, Vydryakova G, Kudryasheva N (2011) Effect of americium-241 on luminous bacteria. Role of peroxides. J Environ Radioact 102:407–411
Antunes MCG, Pereira CCC, Silva JCGE (2007) MCR of the quenching of the EEM of fluorescence of dissolved organic matter by metal ions. Anal Chim Acta 595:9–18
Bironaite D, Anusevic Z, Jacquot JP, Cenas N (1998) Interaction of quinones with Arabidopsis thaliana thioredoxin reductase. Biochem Biophys Acta 1383:82–92
Brunmark A, Cadenas E (1989) Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic Biol Med 7:435–477
Bryantseva NG, Fedorova ES, Sokolova IV, Kudryasheva NS, Khilya VP, Garazd YL (2008) Luminescent analysis of photoinduced detoxification of substituted furocoumarins. J Appl Spectrosc 75:236–240
Bulich AA, Isenberg DL (1981) Use of the luminescent bacterial system for rapid assessment of aquatic toxicity. ISA Trans 20:29–33
Burlakovs J, Kļaviņš M, Osinska L, Purmalis O (2013) The impact of humic substances as remediation agents to the speciation forms of metals in soil. In: APCBEE Procedia. 4th International Conference on Environmental Science and Development–ICESD. 5:192–196
Carlosa L, Mártirea DO, Gonzaleza MC, Gomisb J, Bernabeub A, Amatb AM, Arques A (2012) Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res 46:4732–4740
Chaikovskaya ON, Sokolova IV, Sokolova TV, Yudina NV, Mal’tseva EV, Ivanov AA (2008) Effect of humic acids on phototransformation of methylphenols in water. J Appl Spectrosc 75:597–602
Costerton JW (1988) Structure and plasticity at various organization levels in the bacterial cell. Can J Microbiol 34:513–552
Deprezc K, Robbensd J, Nobelsb I, Vanparysb C, Vanermena G, Tireza K, Michielsc L, Weltens R (2012) DISCRISET: a battery of tests for fast waste classification—application of tests on waste extracts. Waste Manag 32:2218–2228
Deryabin DG, Karimov IF (2010) Characteristics of the response of natural and recombinant luminescent microorganisms in the presence of Fe(2+) ions. Appl Biochem Microbiol 46:28–32
Deryabin DG, Aleshina ES (2008) Natural and recombinant luminescent microorganisms in biotoxicity testing of mineral waters. Appl Biochem Microbiol 44:378–381
Donnelly K, Chen J, Huebner H, Brown K (1997) Utility of four strains of white-rot fungi for the detoxification of 2,4,6-trinitrotoluene in liquid culture. Environ Toxic Chem 16:1105–1110
El’-Registan GI, Mulyukin AL, Nikolaev YA, Suzina NE, Gal’chenko VF, Duda VI (2006) Adaptogenic functions of extracellular autoregulators of microorganisms. Microbiology (Moscow) (Engl Transl) 75:380–389
Esimbekova EN, Kondik AM, Kratasyuk VA (2013) Bioluminescent enzymatic rapid assay of water integral toxicity. Environ Monit Assess 185:5909–5916
Esimbekova EN, Torgashina IG, Kratasyuk VA (2009) Comparative study of immobilized and soluble NADH:FMN-oxidoreductase-luciferase coupled enzyme system. Biochemistry (Mosc) 74:695–700
Esimbekova EN, Kratasyuk VA, Torgashina IG (2007) Disk-shaped immobilized multicomponent reagent for bioluminescent analyses: correlation between activity and composition. Enzyme Microbiol Technol 40:343–346
Fedorova E, Kudryasheva N, Kuznetsov A, Mogil’naya O, Stom D (2007) Bioluminescent monitoring of detoxification processes: activity of humic substances in quinone solutions. J Photochem Photobiol, B 88:131–136
Fedorova ES, Kudryasheva NS, Kuznetsov AM, Stom DI, Belyi AV, Sizykh AG (2005) Detoxication of solutions of organic oxidants by humic substances: bioluminescence monitoring. Doklady Biochem Biophys 403:300–302
Gachter R, Davis JS, Mares A (1978) Regulation of copper availability to phytoplankton by macromolecules in lake water. Environ Sci Technol 12:1416–1421
Gerasimova MA, Kudryasheva NS (2002) Effects of potassium halides on bacterial bioluminescence. J Potochem Photobiol B 66:218–222
Girotti S, Ferri EN, Fumo MG, Maiolini E (2008) Monitoring of environmental pollutants by bioluminescent bacteria. Anal Chim Acta 608:2–21
Gitelzon II, Rodicheva EK, Medvedev SE, Primakova GA, Bartsev SI, Kratasyuk GA, Petushkov VN, Mezhevikin VV, Vysotski ES, Zavoruev VV, Kratasyuk VA (1984) Luminous bacteria. Nauka, Novosibirsk (in Russian)
Grabert E, Kossler F (1997) About the effects of nutrients on the luminescent bacteria test. In: Hastings JW, Kricka LJ, Stanley PE (eds) Bioluminescence and chemiluminescence. Wiley, Chichester, pp 291–294
Granier LK, Lafrance P, Campbell PGC (1999) An experimental design to probe the interactions of dissolved organic matter and xenobiotics: bioavailability of pyrene and 2,2’,5,5’tetrachlorobiphenyl to Daphnia magna. Chemosphere 38:335–350
Gu B, Chen J (2003) Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions. Geochim Cosmochim Acta 67:3575–3582
Hastings JW (2012) Chapter 52—Bioluminescence In: Cell physiology source book. 4th ed. Academic press, pp 925–947
Havelcová M, Mizera J, Sýkorová I, Pekař M (2009) Sorption of metal ions on lignite and the derived humic substances. J Hazard Mater 161:559–564
Hooda V (2007) Phytoremediation of toxic metals from soil and waste water. J Environ Biol 28:367–376
Ilyin LA, Kutsenko SA, Savateev NV, Sofronov GA, Tiunov LA (1990) Toxicological problems in mitigation strategies of chemical industries. J All-Union Mendeleev Chemical Society 35:440–447
Ivask A, Rolova T, Kahru A (2009) A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol 9:1–15
Kamnev AA, Dykman RL, Kovács K, Pankratov AN, Tugarova AV, Homonnay Z, Kuzmann E (2014) Redox interactions between structurally different alkylresorcinols and iron(III) in aqueous media: frozen-solution 57Fe Mössbauer spectroscopic studies, redox kinetics and quantum chemical evaluation of the alkylresorcinol reactivities. Struct Chem 25:649–657
Kamnev AA, Tugarova AV, Selivanova MA, Tarantilis PA, Polissiou MG, Kudryasheva NS (2013) Effects of americium-241 and humic substances on Photobacterium phosphoreum: bioluminescence and diffuse reflectance FTIR spectroscopic studies. Spectrochim Acta A Mol Biomol Spectrosc 100:171–175
Katafias A, Impert O, Kita P (2008) Hydrogen peroxide as a reductant of hexacyanoferrate (III) in alkaline solutions: kinetic studies. Transition Met Chem 33:1041–1046
Keepax R, Jones D, Pepper S, Bryan N (2002) The effects of humic substances upon environmental radioactivity. In: Keith-Roach MJ, Livens FR (ed) Radioactivity in the environment. pp. 143–177
Kirillova TN, Gerasimova MA, Nemtseva EV, Kudryasheva NS (2011) Effect of halogenated fluorescent compounds on bioluminescent reactions. Anal Bioanal Chem 400:343–351
Kirillova TN, Kudryasheva NS (2007) Effect of heavy atom in bioluminescent reactions. Anal Bioanal Chem 387:2009–2016
Kratasyuk VA, Esimbekova EN, Gladyshev MI, Khromichek EB, Kuznetsov AM, Ivanova EA (2001) The use of bioluminescent biotests for study of natural and laboratory aquatic ecosystems. Chemosphere 42:909–915
Kratasyuk VA (1990) Principle of luciferase biotesting. In: Jezowska-Trzebiatowska B (ed) Biolоgical luminescence. World Scientific, Singapore, pp 550–558
Kudryasheva N (2006a) Bioluminescence and exogenous compounds: physicochemical basis for bioluminescence assay. J Photochem Photobiol B 1:77–86
Kudryasheva NS (2006b) Nonspecific effects of exogenous compounds on bacterial bioluminescent enzymes: fluorescence study. Curr Enzyme Inhib 2:363–372
Kudryasheva NS, Nemtseva EV, Kirillova TN (2004) Exogenous compounds in studying the mechanism of electron-excited state formation in bioluminescence. Biopolymers 74:100–104
Kudryasheva NS, Esimbekova EN, Remmel NN, Kratasyuk VA, Visser AJWG, van Hoek A (2003a) Effect of quinones and phenols on the triple-enzyme bioluminescent system with protease. Luminescence 4:224–228
Kudryasheva NS, Nemtseva EV, Visser AJWG, van Hoek A (2003b) Interaction of aromatic compounds with Photobacterium leiognathi luciferase: fluorescence anisotropy study. Luminescence 18:156–161
Kudryasheva N, Vetrova E, Kuznetsov A, Kratasyuk V, Stom D (2002a) Bioluminescent assays: effects of quinones and phenols. Ecotoxicol Environ Saf 53:221–225
Kudryasheva NS, Nemtseva EV, Sizykh AG, Kratasyuk VA, Visser AJ (2002b) Estimation of energy of the upper electron-excited states of the bacterial bioluminescent emitter. J Photochem Photobiol B 68:88–92
Kudryasheva NS (1999) Mechanisms of the effect of xenobiotics on bacterial bioluminescence. Luminescence 14:199–200
Kudryasheva NS, Kudinova IY, Esimbekova EN, Kratasyuk VA, Stom DI (1999a) Effects of quinones and phenols on the NAD(H)-dependent triple systems. Chemosphere 38:751–758
Kydryasheva NS, Zuzikova EV, Gutnik TV (1999b) Mechanism of action of metal salts on bacterial bioluminescent system in vitro. Biofizika 44:244–250
Kudryasheva NS, Kratasyuk VA, Esimbekova EN, Vetrova EV, Kudinova IY, Nemtseva EV (1998) Development of the bioluminescent bioindicators for analyses of pollutions. Field Anal Chem Technol 5:277–280
Kudryasheva NS, Zuzikova EV, Gutnyk TV, Kuznetsov AM (1996) Metallic salts action on bacterial bioluminescent systems of different complexity. Biofizika 41:1264–1269
Kulikova NA, Stepanova EV, Koroleva OV (2005) Mitigating activity of humic substances: direct influence on biota. In: Perminova IV (ed) Use of humic substances to remediate polluted environments: from theory to practice. Springer, The Netherlands, pp 285–309
Kuzminov FI, Brown CM, Fadeev VV, Gorbunov MY (2013) Effects of metal toxicity on photosynthetic processes in coral symbionts, Symbiodinium spp. J Exp Mar Biol Ecol 446:216–227
Lenhart JJ, Cabaniss SE, MacCarthy P, Honeyman BD (2000) Uranium (VI) complexation with citric, humic and fulvic acids. Radiochim Acta 88:345–353
Lorenzo JI, Nieto O, Beiras R (2002) Effect of humic acids on speciation and toxicity of copper to Paracentrotus lividus larvae in seawater. Aquat Toxicol 58:27–41
Ma XY, Wang XC, Ngo HH, Guo W, Wu MN, Wang N (2014) Bioassay based luminescent bacteria: interferences, improvements, and applications. Sci Total Environ 468–469:1–11
Matthiessen A (1996) Kinetic aspects of the reduction of mercury ions by humic substances. Fresenius J Anal Chem 354:747–749
Medvedeva SE, Tyulkova NA, Kuznetsov AM, Rodicheva EK (2009) Bioluminescent bioassays based on luminous bacteria. J Siberian Federal University Biology 2:418–452
Morrisseya R, Hill C, Begley M (2013) Shining light on food microbiology; applications of Lux-tagged microorganisms in the food industry. Trends Food Sci Technol 32:4–15
Natecz-Jawecki G, Rudz B, Sawicki J (1997) Evaluation of toxicity of medical devices using Spirotox and Microtox tests: I. Toxicity of selected toxicants in various diluents. J Biomed Mater Res 35:101–105
Nemtseva EV, Kudryasheva N (2007) The mechanism of electronic excitation in bacterial bioluminescent reaction. Uspekhi Khimii 76:101–102
Oikari A, Kukkonen J, Virtanen V (1992) Acute toxicity of chemicals to Daphnia magna in humic waters. Sci Total Environ 117/118:367–377
Orlov DS (1997) Humic substances in the biosphere. Soros Educ J 2:56–63 (in Russian)
Osterberg R, Shirshova L (1997) Oscillating, nonequilibrium redox properties of humic acids. Geochim Cosmochim Acta 61:4599–4604
Paisio CE, González PS, Gerbaudo A, Bertuzzi ML, Agostini E (2010) Toxicity of phenol solutions treated with rapeseed and tomato hairy roots. Desalination 263:23–28
Park JS, Brown MT, Han T (2012) Phenol toxicity to the aquatic macrophyte Lemna paucicostata. Aquat Toxicol 106–107:182–188
Perminova I, Grechishcheva N, Kovalevskii D, Kudryavtsev A, Petrosyan V, Matorin D (2001) Quantification and prediction of the detoxifying properties of humic substances related to their chemical binding to polycyclic aromatic hydrocarbons. Environ Sci Technol 35:3841–3848
Perminova I, Kovalenko A, Schmitt-Kopplin P, Hatfield K, Hertkorn N, Belyaeva E, Petrosyan V (2005) Design of quinonoid-enriched humic materials with enhanced redox properties. Environ Sci Technol 39:8518–8524
Petukhov VN, Fomchenkov VM, Chugunov VA, Kholodenko VP (2000) Plant biotests for soil and water contaminated with oil and oil products. Appl Biochem Microbiol 36:564–567
Piccolo A, Conte P, Cozzolino A (2001) Chromatographic and spectrophotometric properties of dissolved humic substances compared with macromolecular polymers. Soil Science 166:174–185
Piccolo A (2001) The supramolecular structure of humic substances. Soil Sci 166:810–832
Qua R, Wanga X, Liub Z, Yanb Z, Wang Z (2013) Development of a model to predict the effect of water chemistry on the acute toxicity of cadmium to Photobacterium phosphoreum. J Hazard Mater 262:288–296
Ranjan R, Rastogi NK, Thakur MS (2012) Development of immobilized biophotonic beads consisting of Photobacterium leiognathi for the detection of heavy metals and pesticide. J Hazard Mater 225–226:114–123
Ren S (2003) Phenol mechanism of toxic action classification and prediction: a decision tree approach. Toxicol Lett 144:313–323
Richard C, Guyot G, Trubetskaya O, Trubetskoj O, Grigatti M, Cavan L (2009) Fluorescence analysis of humic-like substances extracted from composts: influence of composting time and fractionation. Environ Chem Lett 7:61–65
Rizzo L (2011) Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res 45:4311–4340
Roda A, Guardigli M, Michelini E, Mirasoni M (2009) Bioluminescence in analytical chemistry and in vivo imaging. Trac-Trends Anal Chem 28:307–322
Roda A, Pasini P, Mirasoni M, Michchelini E, Guardigli M (2004) Biotechnological application of bioluminescence and chemiluminescence. Trends Biotechnol 22:295–303
Rodriguez CE, Shinyashiki M, Froines J, Yu RC, Fukuto JM, Cho AK (2004) An examination of quinone toxicity using the yeast Saccharomyces cerevisiae model system. Toxicol 201:185–196
Rozhko TV, Bondareva LG, Mogilnaya OA, Vydryakova GA, Bolsunovsky AY, Stom DI, Kudryasheva NS (2011) Detoxification of AM-241 solutions by humic substances: bioluminescent monitoring. Anal & Bioanal Chem 400:329–333
Rozhko TV, Kudryasheva NS, Kuznetsov AM, Vydryakova GA, Bondareva LG, Bolsunovsky AY (2007) Effect of low-level α-radiation on bioluminescent assay systems of various complexity. Photochem Photobiol Sci 6:67–70
Sachs S, Bernhard G (2011) Humic acid model substances with pronounced redox functionality for the study of environmentally relevant interaction processes of metal ions in the presence of humic acid. Geoderma 162:132–140
Sakuragi T, Sawa S, Sato S, Kozaki T, Mitsugashira T, Hara M, Suzuki Y (2004) Complexation of americium(III) with humic acid by cation exchange and solvent extraction. Radioanal Nucl Chem 26:309–314
Sanotskiy IV (1970) Methods for determining the toxicity and hazards of chemicals. Medicine, Moscow
Schultz TW, Sinks GD, Cronin MTD (1997) Quinone-induced toxicity to Tetrahymena: structure-activity relationships. Aquat Toxicol 39:267–278
Selivanova MA, Mogilnaya OA, Badun GA, Vydryakova GA, Kuznetsov AM, Kudryasheva NS (2013) Effect of tritium on luminous marine bacteria and enzyme reactions. J Environ Radioact 120:19–25
Shao Y, Wu LL, Gao HW, Wang F (2012) Effect of soluble sulfide on the activity of luminescent bacteria. Molecules 17:6046–6055
Schmeide K, Sachs S, Bernhard G (2012) Np(V) reduction by humic acid: contribution of reduced sulfur functionalities to the redox behavior of humic acid. Sci Total Environ 419:116–123
Shourian M, Noghabi KA, Zahiri HS, Bagheri T, Karballaei G, Mollaei M, Rad I, Ahadi S, Raheb J, Abbasi H (2009) Efficient phenol degradation by a newly characterized Pseudomonas sp. SA01 isolated from pharmaceutical wastewaters. Desalination 246:577–594
Silva JCGE, Machado AASC, Oliveira CJS, Pinto MSSDS (1998) Fluorescence quenching of anthropogenic fulvic acids by Cu(II), Fe(III) and UO2 2+. Talanta 45:1155–1156
Silva JCGE, Machado AASC, Oliveira CJS (1996) Study of the interaction of a soil fulvic acid with UO2 2+ by self-modeling mixture analysis of synchronous molecular fluorescence spectra. Analyst 121:373–379
Skogerboe R, Wilson A (1981) Reduction of ionic species by fulvic acid. Anal Chem 53:228–232
Stasiuk M, Kozubek A (2010) Biological activity of phenolic lipids. Cell Mol Life Sci 67:841–860
Staunton S, Dumat C, Zsolnay A (2002) Possible role of organic matter in radiocaesium adsorption in soils. J Environ Radioact 58:163–173
Stom D, Dagurov A (2004) Cooperation action petroleum products and humic substances on Daphnia magna. Sib Ecol Zh 1:35–40
Stom D (1977) Influence of polyphenols and quinones on aquatic plants and their blocking of SH-groups. J Acta Hydrochim Hydrobiol 5:291–298
Stom DI, Geel TA, Balayan AE, Kuznetsov AM, Medvedeva SE (1992) Bioluminescent method in studying the complex effect of sewage components. Arch Environ Contam Toxicol 22:203–208
Tao S, Liang T, Liu C, Xu S (1999) Uptake of copper by neon tetras (Paracheirodon innesi) in the presence and absence of particulate and humic matter. Ecotoxicoly 8:269–275
Tarasova AS, Kislan SL, Fedorova ES, Kuznetsov AM, Mogilnaya OA, Stom DI, Kudryasheva NS (2012) Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J Photochem Photobiol В 117:164–170
Tarasova AS, Stom DI, Kudryasheva NS (2011) Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. Environ Toxicol Chem 30:1013–1017
Tchaikovskaya O, Sokolova I, Svetlichnyi V, Karetnikova E, Fedorova E, Kudryasheva N (2007) Fluorescence and bioluminescence analysis of sequential UV-biological degradation of p-cresol in water. Luminescence 22:29–34
Tchaikovskaya ON, Karetnikova EA, Sokolova IV, Sokolova TV, Fedorova ES, Kudryasheva NS (2008) Luminescence investigations of the degradation of 2-methylphenol and 4-methylphenol in water. Russian Physics Journal 51:1344–1355
Thakur MS, Ragavan KV (2013) Biosensors in food processing. J Food Sci Technol Mysore 50:625–641
Theng BKG (2012) Chapter 12—humic substances. In: developments in clay science. Formation and Properties of Clay-Polymer Complexes. 4: 391–456
Thomas DJL, Tyrrel SF, Smith R, Farrow S (2009) Bioassays for the evaluation of landfill leachate toxicity. J Toxicol Environ Health 12:83–105
Tigini V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC (2011) Evaluation of toxicity, genotoxicity and environmental risk of simulated textile and tannery wastewaters with a battery of biotests. Ecotoxicol Environ Saf 74:866–873
Trubetskoj OA, Trubetskaya OE, Richard C (2009) Photochemical activity and fluorescence of electrophoretic fractions of aquatic humic matter. Water Resour 36:518–524
Tyulkova NA, Medvedeva SE, Rodicheva EK, Kuznetsov AM (2009) Bacterial bioluminescence and its application. In: Meyer-Rochow VB (ed) Bioluminescence in focus—a collection of illuminating essays. Research Signpost. Trivandrum, India, pp 27–49
Valls M, Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26:327–338
Vetrova EV, Kudryasheva NS, Cheng KH (2009) Effect of quinone on the fluorescence decay dynamics of endogenous flavin bound to bacterial luciferase. J Biophys Chem 141:59–65
Vetrova EV, Kudryasheva NS, Kratasyuk VA (2007) Redox compounds influence on the NAD(P)H:FMN-oxidoreductase-luciferase bioluminescent system. Photochem Photobiol Sci 6:35–40
Vetrova EV, Kudryasheva NS, Visser AJ, van Hoek A (2005) Characteristics of enclogenous flavin fluorescence of Photobacterium leiognathi luciferase and Vibrio fischeri NAD(P)H:FMN-oxidoreductase. Luminescence 20:205–209
Wang W, Lampi MA, Huang XD, Gerhardt K, Dixon DG, Greenberg BM (2009a) Assessment of mixture toxicity of copper, cadmium, and phenanthrenequinone to the marine bacterium Vibrio fischeri. Environ Toxicol 24:166–177
Wang W, Nykamp J, Huang XD, Gerhardt K, Dixon DG, Greenberg BM (2009b) Examination of the mechanism of phenanthrenequinone toxicity to Vibrio fischeri: evidence for a reactive oxygen species-mediated toxicity mechanism. Environ Toxicol Chem 28:1655–1662
Williams PL, James RC, Roberts SM (2000) Principles of toxicology: environmental and industrial applications, 2nd edn. Wiley-Interscience, New York
Yea Z, Zhao Q, Zhang M, Gao Y (2011) Acute toxicity evaluation of explosive wastewater by bacterial bioluminescence assays using a freshwater luminescent bacterium, Vibrio qinghaiensis sp. nov. J Hazard Mater 186:1351–1354
Zhang Y, Zhou J, Lin L, Lin Z (2008) Determination of electrochemical electron-transfer reaction standard rate constants at nanoelectrodes: standard rate constants for ferrocenylmethyltrimethylammonium (III)/(II) and hexacyanoferrate (III)/(II). Electroanal 20:1490–1494
Zhilin D, Schmitt-Kopplin P, Perminova I (2004) Reduction of Cr(VI) by peat and coal humic substances. Environ Chem Lett 2:141–145
Acknowledgments
The work was supported by the Russian Foundation for Basic Research, Grant No.13-04-98072-sibir-a. Part of the work (analysis of detoxification of radioactive solutions) was supported by the Russian Science Foundation, Grant No. 14-14-00076.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kudryasheva, N.S., Tarasova, A.S. Pollutant toxicity and detoxification by humic substances: mechanisms and quantitative assessment via luminescent biomonitoring. Environ Sci Pollut Res 22, 155–167 (2015). https://doi.org/10.1007/s11356-014-3459-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3459-6