Abstract
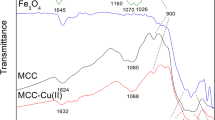
A novel-modified magnetic chitosan adsorbent was used to remove selected pharmaceuticals, i.e., diclofenac (DCF) and clofibric acid (CA) and carbamazepine (CBZ), from aqueous solutions. The characterization of magnetic chitosan was achieved by scanning electron and transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, vibrating sample magnetometer, and nitrogen sorption analysis. The magnetic chitosan had effective sorption affinity for DCF and CA but no sorption of CBZ was observed. The sorption capacities of CA and DCF in the individual solutions were 191.2 and 57.5 mg/g, respectively. While in mixed solution, DCF showed higher sorption affinity. Sorption kinetics indicated a quick equilibrium reached within 2 min. Lower solution pH values were found to be advantageous for the adsorption process. The sorption efficacy of CA declined significantly with increasing inorganic salt concentration. However, sorption performance of DCF was stable under different ionic strength conditions.







Similar content being viewed by others
References
Alkhamis K, Salem M, Khanfar M (2008) The sorption of ketotifen fumarate by chitosan. AAPS PharmSciTech 9(3):866–869. doi:10.1208/s12249-008-9123-z
Bagin V (1967) The chemical remanent magnetization at temperature transitions of lepidocrocite and goethite. Izv Akad Nauk USSR, Fiz Zem 2:104–108
Basta EZ (1959) Some mineralogical relationships in the system Fe 2 O 3-Fe 3 O 4 and the composition of titanomaghemite. Econ Geol 54(4):698–719
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA (2009) Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ Sci Technol 43(3):597–603. doi:10.1021/Es801845a
Beppu MM, Arruda EJ, Vieira RS, Santos NN (2004) Adsorption of Cu(II) on porous chitosan membranes functionalized with histidine. J Membr Sci 240(1–2):227–235. doi:10.1016/j.memsci.2004.04.025
Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 168(2–3):602–608. doi:10.1016/j.jhazmat.2009.02.072
Chai K, Ji H (2012) Dual functional adsorption of benzoic acid from wastewater by biological-based chitosan grafted β-cyclodextrin. Chem Eng J 203:309–318. doi:10.1016/j.cej.2012.07.050
Chang Y-C, Chang S-W, Chen D-H (2006) Magnetic chitosan nanoparticles: studies on chitosan binding and adsorption of Co(II) ions. React Funct Polym 66(3):335–341. doi:10.1016/j.reactfunctpolym.2005.08.006
Colombo U, Gazzarrini F, Lanzavecchia G, Sironi G (1965) Magnetite oxidation: a proposed mechanism. Science 147(3661):1033
Feitknecht W, Lehmann H (1959) Über die Oxydation von Magnetit zu γ‐Fe2O3. Vorläufige Mitt Helv Chim Acta 42(6):2035–2039
Ferrari B, Paxeus N, Lo Giudice R, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac (vol 55, pg 359, 2003). Ecotoxicol Environ Safe 56(3):450. doi:10.1016/S0147-6513(03)00111-8
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Ind Eng Chem Res 37(4):1454–1463. doi:10.1021/ie9703954
Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36(2):357–393
Hirano S, Seino H, Akiyama Y, Nonaka I (1990) Chitosan: a biocompatible material for oral and intravenous administration. Progress in Biomedical Polymers. Plenum Press, New York, pp 283–290
Jansson-Charrier M, Guibal E, Roussy J, Delanghe B, Le Cloirec P (1996) Vanadium (IV) sorption by chitosan: kinetics and equilibrium. Water Res 30(2):465–475
Jjemba PK (2006) Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol Environ Safe 63(1):113–130. doi:10.1016/j.ecoenv.2004.11.011
Juang R-S, Shao H-J (2002) A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res 36(12):2999–3008
Kyzas GZ, Kostoglou M, Lazaridis NK, Lambropoulou DA, Bikiaris DN (2013) Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem Eng J 222:248–258. doi:10.1016/j.cej.2013.02.048
Li GY, Jiang YR, Huang KL, Ding P, Chen J (2008) Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J Alloy Compd 466(1–2):451–456. doi:10.1016/j.jallcom.2007.11.100
Mak S-Y, Chen D-H (2004) Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dyes Pigments 61(1):93–98. doi:10.1016/j.dyepig.2003.10.008
Martucci A, Pasti L, Marchetti N, Cavazzini A, Dondi F, Alberti A (2012) Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater 148(1):174–183. doi:10.1016/j.micromeso.2011.07.009
Mestre AS, Pires J, Nogueira JMF, Carvalho AP (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45(10):1979–1988. doi:10.1016/j.carbon.2007.06.005
Metcalfe CD, Koenig BG, Bennie DT, Servos M, Ternes TA, Hirsch R (2003) Occurrence of neutral and acidic drugs in the effluents of Canadian sewage treatment plants. Environ Toxicol Chem 22(12):2872–2880
Navarro R, Guzmán J, Saucedo I, Revilla J, Guibal E (2003) Recovery of metal ions by chitosan: sorption mechanisms and influence of metal speciation. Macromol Biosci 3(10):552–561. doi:10.1002/mabi.200300013
Ng JCY, Cheung WH, McKay G (2002) Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J Colloid Interface Sci 255(1):64–74. doi:10.1006/jcis.2002.8664
Quinn B, Gagne F, Blaise C (2008) An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Sci Total Environ 389(2–3):306–314. doi:10.1016/j.scitotenv.2007.08.038
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Swaddle TW, Oltmann P (1980) Kinetics of the magnetitemaghemite-hematite transformation, with special reference to hydrothermal systems. Canadian Journal of Chemistry 58(17):1763–1772
Taylor R, Schwertmann U (1974) Maghemite in soils and its origin. Clay Miner 10(4):219–312
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32(11):3245–3260
Tixier C, Singer HP, Oellers S, Muller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37(6):1061–1068. doi:10.1021/Es025834r
Vulliet E, Cren-Olive C, Grenier-Loustalot MF (2011) Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ Chem Lett 9(1):103–114. doi:10.1007/s10311-009-0253-7
Wong YC, Szeto YS, Cheung WH, McKay G (2004) Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process Biochem 39(6):695–704
Wydro P, Krajewska B, Ha̧c-Wydro K (2007) Chitosan as a lipid binder: a Langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules 8(8):2611–2617. doi:10.1021/bm700453x
Zhang Y-L, Zhang J, Dai C-M, Zhou X-F, Liu S-G (2013) Sorption of carbamazepine from water by magnetic molecularly imprinted polymers based on chitosan-Fe3O4. Carbohydr Polym 97(2):809–816. doi:10.1016/j.carbpol.2013.05.072
Zhou XF, Dai CM, Zhang YL, Surampalli R, Zhang T (2011) A preliminary study on the occurrence and behavior of carbamazepine (CBZ) in aquatic environment of Yangtze River Delta, China. Environ Monit Assess 173(1):45–53. doi:10.1007/s10661-010-1369-8
Acknowledgments
This study is financed by Natural Science Foundation of China (21246001, 51138009, 41101480) and the National Key Technologies R&D Program of China (no. 2012BAJ25B02, 2012BAJ25B04).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Roland Kallenborn
Rights and permissions
About this article
Cite this article
Zhang, Y., Shen, Z., Dai, C. et al. Removal of selected pharmaceuticals from aqueous solution using magnetic chitosan: sorption behavior and mechanism. Environ Sci Pollut Res 21, 12780–12789 (2014). https://doi.org/10.1007/s11356-014-3212-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3212-1