Abstract
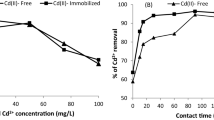
The biosorption characteristics of Cd (II) ions using the living biomass of the marine diatom Phaeodactylum tricornutum were investigated. This microalga is a highly tolerant species to cadmium toxicity; for this reason, it is interesting to know its potential for use in the removal of this metal. The use of living biomass offers better possibilities than that of dead biomass since cadmium can also be bioaccumulated inside the cells. For this purpose, tolerant species are necessary. P. tricornutum is within this category with an EC50,96h of 19.1 ± 3.5 mg Cd (II)/L, and in the present manuscript, it is demonstrated that this microalga has a very good potential for bioremediation of Cd (II) ions in saline habitats. Cadmium removed by the cells was divided into three fractions: total, intracellular and bioadsorbed. The experiments were conducted for 96 h in natural seawater with a concentration range of 1–100 mg Cd (II)/L. Each fraction was characterized every 24 h by sorption isotherms. The experimental isotherm data were analyzed using the Langmuir, Freundlich, Dubinin-Radushkevich and Temkin equations. The biosorption was well described by Langmuir isotherm followed by Freundlich. The worst model was Temkin. The biosorption capacity of this microalga for Cd (II) ions was found to be 67.1 ± 3.2 mg/g after 96 h with approximately 40 % of this capacity in the intracellular fraction. The bioconcentration factor determined was 2,204.7 after 96 h and with an initial Cd (II) concentration of 1 mg/L.








Similar content being viewed by others
References
Abdel-Aty AM, Ammar NS, Abdel Ghafar HH, Ali RK (2013) Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J Adv Res 4:367–374. doi: http://dx.doi.org/10.1016/j.jare.2012.07.004
Ahmady-Asbchin S, Tabaraki R, Jafari N, Allahverdi A, Azhdehakoshpour A (2013) Study of nickel and copper biosorption on brown algae Sargassum angustifolium: application of response surface methodology (RSM). Environ Technol 34:2423–2431
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: effect of temperature. Sep Pur Technol 21:285–294
Bayramoĝlu G, Tüzün I, Çelik G, Yilmaz M, Arica MY (2006) Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int J Miner Process 81:35–43
Bering BP, Dubinin MN, Serpinsky VV (1972) On thermodynamics of adsorption in micropores. J Colloid Interface Sci 38:185–194
Chen CY, Chang HW, Kao PC, Pan JL, Chang JS (2012) Biosorption of cadmium by CO (2)-fixing microalga Scenedesmus obliquus CNW-N. Bioresour Technol 105:74–80. doi:10.1016/j.biortech.2011.11.124
Dixit S, Singh DP (2013) Phycoremediation of lead and cadmium by employing Nostoc muscorum as biosorbent and optimization of its biosorption potential. Int J Phytoremediation 15:801–813
Dönmez GÇ, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34:885–892
Doshi H, Ray A, Kothari IL (2007) Biosorption of cadmium by live and dead Spirulina: IR spectroscopic, kinetics and SEM studies. Curr Microbiol 54:213–218
Dubinin MM, Radushkevich LV (1947) Equation of characteristic curve of activated charcoal. Proc Acad Sci Phys Chem Sect USSR 55:331–333
Folgar S, Torres E, Pérez-Rama M, Cid A, Herrero C, Abalde J (2009) Dunaliella salina as a marine microalga highly tolerant to but a poor remover of cadmium. J Hazard Mater 165:486–493. doi:10.1016/j.jhazmat.2008.10.010
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:385
Gaur N, Flora G, Yadav M, Tiwari A (2013) A review with recent advancements on bioremediation-based abolition of heavy metals. Environ Sci Process Impacts 15:180–193
Gustafsson JP (2013) Visual MINTEQ version 3.1 [online]. Department of Land and Water Resources Engineering, Royal Institute of Technology, Stockholm, Sweden.
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Fund 5:212–223
Hasanuzzaman M, Fujita M (2013) Cadmium: characteristics, sources of exposure, health and environmental effects. In: Chemistry research and applications. Nova Publishers, New York, p 369
Horvatić J, Peršić V (2007) The effect of Ni2+, Co2+, Zn2+, Cd2+ and Hg2+ on the growth rate of marine diatom Phaeodactylum tricornutum Bohlin: microplate growth inhibition test. Bull Environ Contam Toxicol 79:494–498. doi:10.1007/s00128-007-9291-7
Huang F, Dang Z, Guo C-L, Lu G-N, Gu RR, Liu H-J, Zhang H (2013) Biosorption of Cd(II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloid Surface B 107:11–18. doi: http://dx.doi.org/10.1016/j.colsurfb.2013.01.062
Inthorn D, Sidtitoon N, Silapanuntakul S, Incharoensakdi A (2002) Sorption of mercury, cadmium and lead by microalgae. Sci Asia 28:253–261
Karnika AH, Reddy RS, Saradhi SV, Singh J (2007) An eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6:2924–2931
Katırcıo lu H, Aslım B, Rehber Türker A, Atici T, Beyatli Y (2008) Removal of cadmium (II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour Technol 99:4185–4191. doi:10.1016/j.biortech.2007.08.068
Kızılkaya B, Türker G, Akgül R, Doğan F (2011) Comparative study of biosorption of heavy metals using living green algae Scenedesmus quadricauda and Neochloris pseudoalveolaris: equilibrium and kinetics. J Dispers Sci Technol 33:410–419. doi:10.1080/01932691.2011.567181
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lezcano JM, Gonzalez F, Ballester A, Blazquez ML, Munoz JA, Garcia-Balboa C (2011) Sorption and desorption of Cd, Cu and Pb using biomass from an eutrophized habitat in monometallic and bimetallic systems. J Environ Manag 92:2666–2674. doi:10.1016/j.jenvman.2011.06.004
López-Chuken UJ, Young SD, Sánchez-González MN (2010) The use of chloro-complexation to enhance cadmium uptake by Zea mays and Brassica juncea: testing a “free ion activity model” and implications for phytoremediation. Int J Phytoremediation 12:680–696. doi:10.1080/15226510903353161
Lourie E, Gjengedal E (2011) Metal sorption by peat and algae treated peat: kinetics and factors affecting the process. Chemosphere 85:759–764. doi:10.1016/j.chemosphere.2011.06.055
Maret W, Moulis JM (2013) The bioinorganic chemistry of cadmium in the context of its toxicity. Met Ions Life Sci 11:1–29. doi:10.1007/978-94-007-5179-8_1
Matsunaga T, Takeyama H, Nakao T, Yamazawa A (1999) Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J Biotechnol 70:33–38
Michalak I, Chojnacka K, Witek-Krowiak A (2013) State of the art for the biosorption process—a review. Appl Biochem Biotechnol 170:1389–1416. doi:10.1007/s12010-013-0269-0
Millero FJ, Feistel R, Wright DG, McDougall TJ (2008) The composition of standard seawater and the definition of the reference-composition salinity scale. Deep-Sea Res PT I 55:50–72. doi: http://dx.doi.org/10.1016/j.dsr.2007.10.001
Monteiro CM, Brandao TR, Castro PM, Malcata FX (2012) Modelling growth of, and removal of Zn and Hg by a wild microalgal consortium. Appl Microbiol Biotechnol 94:91–100. doi:10.1007/s00253-011-3826-x
Olguín EJ, Sánchez-Galván G (2012) Heavy metal removal in phytofiltration and phycoremediation: the need to differentiate between bioadsorption and bioaccumulation. New Biotechnol 30:3–8. doi: http://dx.doi.org/10.1016/j.nbt.2012.05.020
Pérez-Rama M, Torres E, Suárez C, Herrero C, Abalde J (2010) Sorption isotherm studies of Cd (II) ions using living cells of the marine microalga Tetraselmis suecica (Kylin) Butch. J Environ Manag 91:2045–2050. doi:10.1016/j.jenvman.2010.05.014
Rajamani S, Siripornadulsil S, Falcao V, Torres M, Colepicolo P, Sayre R (2007) Phycoremediation of heavy metals using transgenic microalgae. Adv Exp Med Biol 616:99–109. doi:10.1007/978-0-387-75532-8_9
Sarı A, Tuzen M (2008) Biosorption of cadmium (II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157:448–454. doi: http://dx.doi.org/10.1016/j.jhazmat.2008.01.008
Sekabira K, Origa HO, Basamba TA, Mutumba G, Kakudidi E (2011) Application of algae in biomonitoring and phytoextraction of heavy metals contamination in urban stream water. Int J Environ Sci Technol 8:115–128. doi:10.1007/BF03326201
Singh SN, Tripathi RD (2007) Environmental bioremediation technologies. Springer, Berlin Heidelberg
Tangaromsuk J, Pokethitiyook P, Kruatrachue M, Upatham ES (2002) Cadmium biosorption by Sphingomonas paucimobilis biomass. Bioresour Technol 85:103–105
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim, URSS 12:217–222
Torres E, Cid A, Fidalgo P, Herrero C, Abalde J (1995) Tolerance and detoxification mechanisms in marine diatom Phaeodactylum tricornutum exposed to cadmium. J Mar Biotechnol 3:176–178
Torres E, Cid A, Fidalgo P, Herrero C, Abalde J (1997) Long-chain class III metallothioneins as a mechanism of cadmium tolerance in the marine diatom Phaeodactylum tricornutum Bohlin. Aquat Toxicol 39:231–246
Torres E, Cid A, Herrero C, Abalde J (1998) Removal of cadmium ions by the marine diatom Phaeodactylum tricornutum Bohlin accumulation and long-term kinetics of uptake. Bioresour Technol 63:213–220
Torres E, Mera R, Abalde J (2013) Toxicity and tolerance in microalgal cells exposed to cadmium: a current overview. In: Hasanuzzaman M, Fujita M (eds) Cadmium: characteristics, sources of exposure, health and environmental effects. Chemistry research and applications. Nova Publishers, New York, pp 171–196
Tüzün I, Bayramoĝlu G, Yalçin E, Başaran G, Çelik G, Arica Y (2005) Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage 77:85–92
Volesky B (1990) Biosorption of heavy metals, vol M-29. CRC Press, Inc., Boca Raton
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216
Yan H, Yang L, Wang Q (2011) Evaluation of cadmium species lability using ion-pair reversed phase HPLC coupled on-line with inductively coupled plasma mass spectrometry. Talanta 84:287–292. doi:10.1016/j.talanta.2011.01.019
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Torres, E., Mera, R., Herrero, C. et al. Isotherm studies for the determination of Cd (II) ions removal capacity in living biomass of a microalga with high tolerance to cadmium toxicity. Environ Sci Pollut Res 21, 12616–12628 (2014). https://doi.org/10.1007/s11356-014-3207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3207-y