Abstract
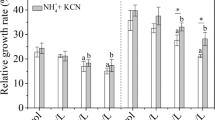
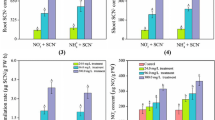
Responses of free amino acids to botanical assimilation of free cyanide were investigated. Young rice seedlings (Oryza sativa L. cv. XZX 45) were grown in nutrient solution amended with free cyanide (KCN). Cyanide was analyzed in solution as well as in plant materials to estimate the phyto-assimilation potential. Free amino acids in different parts of plants were also measured to determine metabolic responses to KCN exposure. Phyto-assimilation of KCN was obvious, and the rates were positively correlated to the concentration supplied. Although changes in total amino acid content in plant materials were negligible during KCN metabolism (p > 0.05), responses of different amino acids to KCN treatments were quite different. All treatments with KCN increased the content of proline (Pro) and isoleucine (Ile) in roots significantly compared with control (p < 0.05), while changes of aspartic acid, lysine, and histidine in roots were more evident at higher KCN treatments (p < 0.05). Results indicate that the content of Pro, Ile, and tyrosine showed pronounced increase in shoots of rice seedlings exposed to KCN at 1.44 mg CN/L or higher (p < 0.05). Other amino acids slightly changed in all plant materials exposed to KCN (p > 0.05). Results indicate that specific amino acids in rice seedlings showed positive response to non-toxic concentrations of exogenous KCN. These findings could provide additional insights into the inducible mechanisms underlying the involvement of amino acids in KCN metabolism.




Similar content being viewed by others
References
Binder S, Knill T, Schuster J (2007) Branched-chain amino acid metabolism in higher plants. Physiol Plant 129:68–78
Cuin TA, Shabala S (2007) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225:753–761
Delfine S, Alvino A, Villani MC, Loreto F (1999) Restrictions to carbon dioxide conductance and photosynthesis in spinach leaves recovering from salt stress. Plant Physiol 119:1101–1106
Di Martino C, Delfine S, Pizzuto R, Loreto F, Fuggi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158:455–463
Ebbs SD (2004) Biological degradation of cyanide compounds. Curr Opin Biotech 15:231–236
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytorem 10:343–357
Ebbs SD, Kosma D, Nielson EH, Machingura M, Baker AJM, Woodrow IE (2010) Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.). Plant Cell Environ 33:1152–1160
Goudey JS, Tittle FL, Spencer MS (1989) A role for ethylene in the metabolism of cyanide by higher plants. Plant Physiol 89:1306–1310
Hamilton EW, Heckathorn SA (2001) Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol 126:1266–1274
Hasegawa R, Maruyama A, Sasaki H, Tada T, Esashi Y (1995) Possible involvement of ethylene-activated β-cyanoalanine synthase in the regulation of cocklebur seed germination. J Exp Bot 46:551–556
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 55:373–399
Joshi V, Joung JG, Fei ZJ, Jander G (2010) Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino acids 39:933–947
Larsen M, Ucisik A, Trapp S (2005) Uptake, metabolism, accumulation and toxicity of cyanide in willow trees. Environ Sci Technol 39:2135–2142
Lea PJ, Sodek L, Parry MAJ, Shewry PR, Halford NG (2007) Asparagine in plants. Ann Appl Biol 150:1–26
Liang WS (2003) Drought stress increases both cyanogenesis and beta-cyanoalanine synthase activity in tobacco. Plant Sci 165:109–1115
Kao CM, Liu JK, Lou HR, Lin CS, Chen SC (2003) Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 50:1055–1061
Machingura M, Ebbs S (2010) Increased beta-cyanoalanine synthase and asparaginase activity in nitrogen-deprived wheat exposed to cyanide. J Plant Nutr Soil Sci 173:808–810
Machingura M, Sidibe A, Wood AJ, Ebbs SD (2013) The beta-cyanoalanine pathway is involved in the response to water deficit in Arabidopsis thaliana. Plant Physiol Biochem 33:159–169
Manning K (1988) Detoxification of cyanide by plants and hormone action. In: Ciba Foundation (ed) Cyanide compounds in biology. John Wiley & Sons, Chichester, pp 92–110
Maruyama A., Yoshiyama M., Adachi Y., Nanba H., Hasegawa R., Esashi Y. (1997) Possible participation of beta-cyanoalanine synthase in increasing the amino acid pool of cocklebur seeds in response to ethylene during the pre-germination period. Aust J Plant Physiol 24:751–757
Maruyama A, Saito K, Ishizawam K (2001) β-cyanoalanine synthase and cysteine synthase from potato: molecular cloning, biochemical characterization, and spatial and hormonal regulation. Plant Mol Biol 46:749–760
Paungfoo-Lonhienne C, Lonhienne TGA, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S (2008) Plants can use protein as a nitrogen source without assistance from other organisms. Proc Natl Acad Sci USA 105:4524–4529
Peiser GD, Wang TT, Hoffman NE, Yang SF, Walsh CT (1984) Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carbonxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA 81:3059–3063
Rai VK (2002) Role of amino acids in plant responses to stresses. Biol Plant 45:481–487
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulphonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Sakamoto A, Murata N (2000) Genetic engineering of glycine betaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51:81–88
Siegien I, Bogatek R (2006) Cyanide action in plants—from toxic to regulatory. Acta Physiol Plant 28:483–497
Tang P, Hseu YC, Chou HH, Huang KY, Chen SC (2010) Proteomic analysis of the effect of cyanide on Klebsiella oxytoca. Curr Microbiol 60:224–228
Wang JF, Dong CX, Shen QR (2007) Effect of ammonium/nitrate ratios on the free amino acids and three kinds of enzymes of nitrogen metabolism in spinach shoot. Plant Nutrition Fertilizer Sci 13:664–670 (In Chinese)
Yoshiba Y, Kiyosue T, Nakashima K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38:1095–1102
Yu XZ, Trapp S, Zhou PH, Wang C, Zhou XS (2004) Metabolism of cyanide by Chinese vegetation. Chemosphere 56:121–126
Yu XZ, Trapp S, Zhou PH (2005) Phytotoxicity of cyanide to weeping willows. Environ Sci Pollut Res 12:109–113
Yu XZ, Lu PC, Yu Z (2012) On the role of beta-cyanoalanine synthase (CAS) in metabolism of free cyanide and ferri-cyanide by rice seedlings. Ecotoxicology 21:548–556
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Cupper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. J Agri Food Chem 57:411–419
Zhang PP, Fu JM, Hu LX (2012) Effects of alkali stress on growth, free amino acids and carbohydrates metabolism in Kentucky bluegrass (Poa pratenis). Ecotoxicology 21:1911–1918
Zhao FG, Sun C, Liu YL (2001) Ornithine pathway in proline biosynthesis activated by salt stress in barley seedlings. Acta Botanica Sinica 43:35–40
Acknowledgments
This work is financially supported by a research foundation from Guilin University of Technology (grant no: GUTRC2011007) and a grant from Pearl River Water Resources Protection Bureau.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Yu, XZ., Zhang, XH. & Liu, W. Responses of free amino acids in rice seedlings during cyanide metabolism. Environ Sci Pollut Res 21, 1411–1417 (2014). https://doi.org/10.1007/s11356-013-2034-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2034-x