Abstract
Purpose
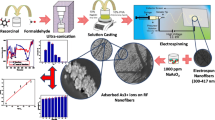
Nanomaterials such as iron oxides and ferrites have been intensively investigated for water treatment and environmental remediation applications. The purpose of this work is to synthesize α-Fe2O3 nanofibers for potential applications in removal and recovery of noxious Cr(VI) from wastewater.
Methods
α-Fe2O3 nanofibers were synthesized via a simple hydrothermal route followed by calcination. The crystallographic structure and the morphology of the as-prepared α-Fe2O3 nanofibers were characterized by X-ray diffraction, scanning electron microscope, and transmission electron microscope. Batch adsorption experiments were conducted, and Fourier transform infrared spectra were recorded before and after adsorption to investigate the Cr(VI) removal performance and adsorption mechanism. Langmuir and Freundlich modes were employed to analyze the adsorption behavior of Cr(VI) on the α-Fe2O3 nanofibers.
Results
Very thin and porous α-Fe2O3 nanofibers have been successfully synthesized for investigation of Cr(VI) removal capability from synthetic wastewater. Batch experiments revealed that the as-prepared α-Fe2O3 nanofibers exhibited excellent Cr(VI) removal performance with a maximum adsorption capacity of 16.17 mg g−1. Furthermore, the adsorption capacity almost kept unchanged after recycling and reusing. The Cr(VI) adsorption process was found to follow the pseudo-second-order kinetics model, and the corresponding thermodynamic parameters ΔG°, ΔH°, and ΔS° at 298 K were calculated to be −26.60 kJ mol−1, −3.32 kJ mol−1, and 78.12 J mol−1 K−1, respectively.
Conclusions
The as-prepared α-Fe2O3 nanofibers can be utilized as efficient low-cost nano-absorbents for removal and recovery of Cr(VI) from wastewater.








Similar content being viewed by others
References
Ai ZH, Cheng Y, Zhang LZ, Qiu JR (2008) Efficient removal of Cr(VI) from aqueous solution with Fe@Fe2O3 core-shell nanowires. Environ Sci Technol 42:6955–6960
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purif Rev 39:95–171
Aravindhan R, Madhan B, Rao JR, Nair BU, Ramasami T (2004) Bioaccumulation of chromium from tannary wastewater: an approach for chrome recovery and reuse. Environ Sci Technol 38:300–306
Benito Y, Ruiz ML (2002) Reverse osmosis applied to metal finishing wastewater. Desalination 142:229–234
Cao CY, Cui ZM, Chen CQ, Song WG, Cai W (2010) Ceria hollow nanospheres produced by a template-free microwave assisted hydrothermal method for heavy metal ion removal and catalysis. J Phys Chem C 114:9865–9870
Carmona MER, Da Silva MAP, Leite SG (2005) Biosorption of chromium using factorial experimental design. Process Biochem 40:779–788
Dupont L, Guillon E (2003) Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ Sci Technol 37:4235–4241
El-Mouzdahir Y, Elmchaouri A, Mahboub R, Gil A, Korili SA (2007) Adsorption of methylene blue from aqueous solutions on a Moroccan clay. J Chem Eng Data 52:1621–1625
Gou XL, Li R, Wang GX, Chen Z, Wexler D (2009) Room-temperature solution synthesis of Bi2O3 nanowires for gas sensing application. Nanotechnology 20:495501
Güell R, Anticó E, Salvadó V, Fontàs C (2008) Efficient hollow fiber supported liquid membrane system for the removal and preconcentration of Cr(VI) at trace levels. Sep Purif Technol 62:389–393
Gupta VK, Carrott PJM, Carrott MML, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solution by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modeling of cadmium(II) biosorption by nonliving algal biomass Odedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi (2008c) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloid Surface B 64:170–178
Gupta VK, Singh AK, Gupta B (2007) Schiff bases as cadmium(II) selective ionophores in polymeric membrane electrodes. Anal Chim Acta 583:340–348
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminum industry waste. Water Res 35:1125–1134
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271:321–328
Gupta VK, Rastogi A (2008d) Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Agarwal S, Saleh TA (2011) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45:2207–2212
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Humplik T, Lee J, O’Hern S, Fellman BA et al (2011) Nanostructured materials for water desalination. Nanotechnology 22:292001
Kobya M (2004) Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresour Technol 91:317–321
Li J, Lai XY, Xing CJ, Wang D (2010) One-pot synthesis of porous hematite hollow microspheres and their application in water treatment. J Nanosci Nanotechnol 10:7707–7710
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J Hazard Mater 137:762–811
Mor S, Ravindra K, Bishnoi NR (2007) Adsorption of chromium from aqueous solution by activated alumina and activated charcoal. Bioresour Technol 98:954–957
Narayan R (2010) Use of nanomaterials in water purification. Mater Today 13:44–46
Sağ Y, Aktay Y (2002) Kinetic studies on sorption of Cr(VI) and Cu(II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem Eng J 12:143–153
Samaratunga SS, Nishimoto J, Tabata (2008) Extraction of chromium(VI) by salting-out with a homogeneous, mixed solvent of water and 2-propanol: a laboratory study. Environ Sci Pollut Res 15:27–30
Sharma YC, Srivastava V, Singh VK, Kaul SN, Weng CH (2009) Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30:583–609
Srivastava SK, Gupta VK, Jain S (1995) Determination of lead using a poly(vinyl chloride)-based crown ether membrane. Analyst 120:495–498
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Environ Eng 123:461–468
Tang W, Li Q, Gao S, Shang JK (2011) Arsenic(III,V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J Hazard Mater 192:131–138
Wang P, Lo IMC (2009) Synthesis of mesoporous magnetic γ-Fe2O3 and its application to Cr(VI) removal from contaminated water. Water Res 43:3727–3734
Xing YQ, Chen XM, Wang DW (2007) Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ Sci Technol 41:1439–1443
Zhong LS, Hu JS, Liang HP, Cao AM, Song WG, Wan LJ (2006) Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv Mater 18:2426–2431
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51071131), Program for New Century Excellent Talents in University (NCET-10-0890), the Scientific Research Foundation for the Returned Overseas Chinese Scholars ([2008]488), and the Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (10CSPC-1-7). We would like to express our gratitude to Prof. Gupta for his suggestion for improving this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Ren, T., He, P., Niu, W. et al. Synthesis of α-Fe2O3 nanofibers for applications in removal and recovery of Cr(VI) from wastewater. Environ Sci Pollut Res 20, 155–162 (2013). https://doi.org/10.1007/s11356-012-0842-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0842-z