Abstract
Purpose
The purpose of the research is to investigate the applicability of the low-cost natural biosorbents for the removal of Pb(II) ions from aqueous solution and effluent from battery industry.
Methods
Six different biosorbents namely rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots have been used for the removal of Pb(II) ions from aqueous solution in batch process. All the biosorbents were collected from local area near Kolkata, West Bengal, India. The removal efficiency was determined in batch experiments for each biosorbent.
Results
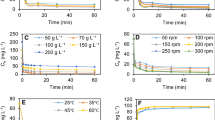
The biosorbents were characterized by SEM, FTIR, surface area, and point of zero charge. The sorption kinetic data was best described by pseudo-second-order model for all the biosorbents except rice husk which followed intraparticle diffusion model. Pb(II) ions adsorption process for rice straw, rice bran, and hyacinth roots were governed predominately by film diffusion, but in the case of rice husk, it was intraparticle diffusion. Film diffusion and intraparticle diffusion were equally responsible for the biosorption process onto coconut shell and neem leaves. The values of mass transfer coefficient indicated that the velocity of the adsorbate transport from the bulk to the solid phase was quite fast for all cases. Maximum monolayer sorption capacities onto the six natural sorbents studied were estimated from the Langmuir sorption model and compared with other natural sorbents used by other researchers. The Elovich model, the calculated values of effective diffusivity, and the sorption energy calculated by using the Dubinin–Radushkevich isotherm were indicated that the sorption process was chemical in nature. The thermodynamic studies indicated that the adsorption processes were endothermic. FTIR studies were carried out to understand the type of functional groups responsible for Pb(II) ions binding process. Regeneration of biosorbents were carried out by desorption studies using HNO3. Battery industry effluents were used for the application study to investigate applicability of the biosorbents.
Conclusion
The biosorbents can be utilized as low-cost sorbents for the removal of Pb(II) ions from wastewater.










Similar content being viewed by others
Abbreviations
- a 1 :
-
Elovich constant which gives an idea of the reaction rate constant
- b :
-
Langmuir constant (in liters per milligram)
- B :
-
Time-dependent factor
- b 1 :
-
Elovich constants and represents the rate of chemisorption at zero coverage
- C :
-
Intraparticle diffusion constant
- C a :
-
Pb(II) ions concentration on the sorbent at equilibrium (in milligrams per liter)
- Cabs :
-
The amount of Pb(II) adsorbed onto sorbent surface (in moles per gram)
- C e :
-
Pb(II) ions concentration in solution at equilibrium (in milligrams per liter)
- C 0 :
-
Initial Pb(II) ions concentration (in milligrams per liter)
- C t :
-
Pb(II) ions concentration at time t (in milligrams per liter)
- D e :
-
Effective diffusion coefficient of adsorbates in the sorbent phase (in square meters per second)
- E :
-
Mean sorption energy (in kilojoules per mole)
- F(t):
-
Ratio of amount of Pb(II) ions adsorbed per gram of sorbent at any time to that of at equilibrium time
- ∆G 0 :
-
Gibbs free energy (in kilojoules per mole)
- ∆H 0 :
-
Enthalpy (in kilojoules per mole)
- K 1 :
-
Lagergren rate constant (per minute)
- K 2 :
-
Pseudo-second-order rate constant (in milligrams per gram per minute)
- K i :
-
Intraparticle rate constant (in milligrams per gram per square root of minute)
- K bq :
-
The constant obtained by multiplying q max and b
- \( K_c^0 \) :
-
Thermodynamic equilibrium constant
- \( {K\prime_c} \) :
-
Apparent equilibrium constant
- M :
-
Mass of the sorbent per unit volume (in grams per liter)
- m s :
-
Amount of sorbent added in gram
- n :
-
Freundlich constants intensity of sorption (in milligrams per gram)/(in milligrams per liter)1/n
- n 1 :
-
An integer
- q e :
-
Amount adsorb per gram of the sorbent at equilibrium
- q max :
-
Maximum sorption capacity (in milligrams per gram)
- q t :
-
Amount (in milligrams) adsorb per gram of sorbent
- q tm :
-
Amount (in milligrams) adsorb per gram of sorbent from model
- \( q_{ \propto } \) :
-
Amount (in milligrams) adsorb per gram of sorbent at infinite time
- r 2 :
-
Correlation coefficient
- R :
-
Ideal gas constant in kilojoules per mole per kelvin
- R L :
-
Separation factor
- R a :
-
Radius of the sorbent particle (in meter)
- S S :
-
External surface area of the sorbent per unit volume (per meter)
- ∆S 0 :
-
Entropy (in kilojoules per mole kelvin)
- t :
-
Time (in minutes)
- T:
-
Temperature in kelvin
- TDS:
-
Total dissolves solid
- t 0 :
-
Elovich constant equals to 1/(a 1·b 1)
- V :
-
Volume of the solution (in liters)
- X m :
-
Maximum sorption capacity of sorbent (in millimoles per gram)
- β :
-
Mass transfer coefficient (in centimeters per second)
- λ :
-
Constant related to sorption energy (in square moles per square kilojoule)
- ε :
-
Polanyi potential (in square kilojoules per square mole)
- \( \chi_t^2 \) :
-
Chi-square value (\( \chi_t^2 = \sum {\frac{{{{({q_t} - {q_{{tm}}})}^2}}}{{{q_{{tm}}}}}} \))
Reference
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Separ Purif Rev 39:95–171
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
APHA, AWWA, WEF (1998) Standard methods for examination of water and wastewater, 20th edn. APHA, New York
Bhattacharya AK, Naiya TK, Mandal SN, Das SK (2008) Adsorption, kinetics and equilibrium studies on the removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137(3):529–541
Blazquez G, Calero M, Hernainz F, Tenorio G, Martin-Lara MA (2010) Equilibrium biosorption of lead(II) from aqueous solutions by solid waste from. Chem Eng J 160:615–622
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetic. J Am Chem Soc 69:2836–2848
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solutions by hazelnut shell. Water Res 34:2955–2962
Conrad K, Hansen HCB (2007) Sorption of zinc and lead on coir. Bioresour Technol 98:89–97
Cruz-Olivares J, Pérez-Alonso C, Barrera-Diaz C, Lَpez G, Balderas-Hernandez P (2010) Inside the removal of lead(II) from aqueous solutions by De-Oiled Allspice Husk in batch and continuous processes. J Hazard Mater 181:1095–1101
Dahiya S, Tripathi RM, Hegde AG (2008) Biosorption of lead and copper from aqueous solutions by pre-treated crab and arca shell biomass. Bioresour Technol 99:179–187
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons I. Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii 21:1351–1362
Freundlich H (1906) Adsorption in solution. PhysChemie 57:384–410
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 27:321–328
Gupta VK, Andimranali (2008) Removal of endosulfan and methoxychlor from water on carbonslurry. Environ Sci Technol 42:766–770
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloids Surf B Biointerfaces 64:170–178
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash: a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Separ Sci Tech 32(17):2883–2912
Gupta VK, Mohan D, Sharma S (1998) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Separ Sci Tech 33:1331–1343
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Singh P, Rahman N (2004) Adsorption behavior of Hg(II), Pb(II) and Cd(II) from aqueous solution on Duolite C-433: a synthetic resin. J Colloid Interface Sci 275:398–402
Gupta VK, Ali I, Saini VK (2007) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Carrott PJM, Carrott MMLR, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Tech 39:783–842
Gupta VK, Rastogi A, Nayak A (2010a) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Rastogi A, Nayak A (2010b) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Han R, Zhang J, Zou W, Shi J, Lui H (2005) Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater 125:266–271
Hasana SH, Srivastavaa P, Talatb M (2010) Biosorption of lead using immobilized Aeromonas hydrophila biomass in up flow column system: factorial design for process optimization. J Hazard Mater 177:312–322
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper(II) onto peat. Adsorp Sci Technol 20(8):797–815
Ho YS, Mckay G, Wase DAJ, Foster CF (2000) Study of the sorption of divalent metal on to peat. Adsorp Sci Technol 18:639–650
Imamoglu M, Tekir O (2008) Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 228:108–113
IS 10500 (1992) Drinking water specification (reaffirmed 1993). http://www.hppcb.nic.in/EIAsorang/Spec.pdf. Accessed 09 Aug 2007
Kalavathy MH, Karthikeyan T, Rajgopal S, Miranda LR (2005) Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J Colloid Interface Sci 292:354–362
Khalid LB, Girgis BS, Tawfik T (2001) Decomposition of H2O2 on activated carbon obtained from olive stones. J Chem Technol Biotechnol 76:132–1140
Khan TA, Singh V, Ali I (2009) Sorption of Cd(II), Pb(II), and Cr(VI) metal ions from wastewater using bottom fly ash as a low cost sorbent. J Environ Protect Sci 3:124–132
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39
Lalhruaitluanga H, Jayaram K, Prasad MNV, Kumar KK (2010) Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)—a comparative study. J Hazard Mater 175:311–318
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lee SH, Jung CH, Chung H, Lee MY, Yang JW (1998) Removal of heavy metals from aqueous solution by apple residues. Process Biochem 33:205–211
Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solutions by saw dust and modified peanut husk. J Hazard Mater 141:163–167
Liao D, Zheng W, Li X, Yang Q, Yue X, Guo L, Zeng G (2010) Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J Hazard Mater 177:126–130
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 60:267–312
Low KS, Lee CK, Liew SC (2000) Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem 36:59–64
Mahmoud M, Osman MM, Hafez OF, Hegazi AH, Elmelegy E (2010) Removal and pre concentration of lead (II) and other heavy metals from water by alumina adsorbents developed by surface-adsorbed-dithizone. Desalination 251:123–130
Mckay G, Otterburn MS, Sweeney AG (1981) Surface mass transfer processes during colour removal from effluent using silica. Water Res 15:327–331
Mckay G, Blair HS, Gardener JR (1982) Adsorption of dyes on chitin. I. Equilibrium studies. J Appl Polym Sci 27:3043–3057
Meunier N, Laroulandie J, Blais JF, Tyagi RD (2003) Cocoa shells for heavy metal removal from acidic solutions. Bioresour Technol 90:255–263
Naiya TK, Bhattacharya AK, Das SK (2009a) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interface Sci 333:14–26
Naiya TK, Bhattacharya AK, Mandal SN, Das SK (2009b) The sorption of lead(II) ions on rice husk ash. J Hazard Mater 163:1254–1264
Pehlivan E, Altun T, Parlayici S (2009) Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J Hazard Mater 164:982–986
Qin LH, Zhang FB, Zang GL, Zhang SH (2007) Kinetics and mechanism of the sorption of NTS macroporous adsorption resin. Chem Industry Eng 24(3):245–248
Reddy DHK, Harinatha Y, Seshaiah K, Reddy AVR (2010) Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597
Saeeda A, Iqbala M, Akhtarb MW (2005) Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater B117:65–73
Sawalha MF, Peralta-Videa JR, Romero-Gonzalez J, Gardea-Torresdey JL (2006) Biosorption of Cd(II), Cr(III), and Cr(VI) by saltbush (Atriplex canescens) biomass: thermodynamic and isotherm studies. J Colloid Interface Sci 300:100–104
Seeber G, Buchmeiser MR, Bonn GK, Bertsch T (1998) Determination of airborne, volatile amines from polyurethane foams by sorption onto a high-capacity cation-exchange resin based on poly (succinic acid). J Chromatogr A 809(1–2):121–129
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Environ Eng 123:461–468
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater B134:257–267
Srivastava VC, Mall ID, Mishra IM (2009) Competitive adsorption of cadmium(II) and nickel(II) ions from aqueous solution onto rice husk ash. Chem Eng Proc 48(1):370–379
Teng H, Hsieh CT (1999) Activation energy for oxygen chemisorption on carbon at low temperature. Ind Eng Chem Res 38:292–297
Unlu N, Ersoz M (2007) Removal of heavy metal ions by using dithiocarbamated-sporopollenin. Separ Purif Tech 52:461–469
Vermeulen T (1953) Theory for irreversible and constant pattern solid diffusion. Ind Eng Chem 45(8):1664–1670
Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol 101:5808–5814
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Webi TW, Chakravort RK (1974) Pore and solid diffusion models for fixed bed absorbers. AICHE J 20:228–238
Zaki AB, El-Sheikh MY, Evans J, EI-Safty SA (2000) Kinetics and mechanism of the sorption of some aromatic amines onto amberlite IRA-904 anion-exchange resin. J Colloid Interface Sci 221:58–63
Acknowledgment
The authors acknowledge DST, Govt. of India for the financial support for the project work and fellowship to Mr. B. Singha (file no. DST/WTI/2K9/141, dated 19.05.2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Singha, B., Das, S.K. Removal of Pb(II) ions from aqueous solution and industrial effluent using natural biosorbents. Environ Sci Pollut Res 19, 2212–2226 (2012). https://doi.org/10.1007/s11356-011-0725-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0725-8