Abstract
Purpose
The aim of the current study was to further investigate the concept of previously reported high occurrence of comorbidities in obstructive sleep patients (OSA) with insomnia-like symptoms. We hypothesized that this finding at least partly is mediated by nocturnal hypoxia. Moreover, we speculated that the spectrum of the clinical OSA phenotypes differs between European geographical regions.
Methods
Cohort of the European Sleep Apnea Database (n = 17,325; 29.9% females) was divided into five subcohorts according to geographical region (North, East, South, West, Central) and further into four clinical presentation phenotypes based on daytime symptoms (EDS) and characteristics suggestive of insomnia.
Results
The insomnia phenotype (alone or together with EDS) dominated in all European regions. Isolated insomnia, however, was less common in the West. Insomnia phenotype was associated with the highest proportion of cardiovascular comorbidity (51.7% in the insomnia vs. 43.9% in the EDS type). Measures of nocturnal hypoxemia were independently associated with cardiovascular comorbidity in phenotypes with insomnia-like symptoms. The burden of comorbidities was high across all geographical regions and clinical phenotypes. Regional differences were clinically relevant for age (48 vs. 54 years), BMI (29 vs. 34 kg/m2), and ODI (15 vs. 32/h).
Conclusion
High prevalence of particularly cardiovascular comorbidity among patients with insomnia-like symptoms was linked to nocturnal hypoxemia. Considerable differences in clinical presentation were found among OSA patients across Europe. Our data underline that physicians should ask their patients with suspected OSA also for insomnia symptoms. It remains to be explored if a reduction of nocturnal hypoxemia predicts the improvement of insomnia symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a heterogeneous, complex disorder encompassing a wide variety of symptoms and disorders. A good understanding of the comorbidities and therapeutic outcome in various clinical phenotypes improves the possibility to provide a targeted therapy in each individual patient. We have previously reported on four clinical phenotypes defined by daytime and nighttime symptoms that differ in terms of burden of comorbidity [1]. A phenotype associated with insomnia symptoms was linked to a higher prevalence of cardiovascular diseases (CVD) and other common disorders [1, 2] in a manner that was not explained by the severity of OSA.
The underlying mechanism behind the increased prevalence of cardiovascular comorbidity in the insomnia-like OSA phenotype remains unexplained but some observations have been reported. For instance, patients of the insomnia phenotype were generally older [1]. There are also data suggesting a higher sympathetic activity [3] and association of cardiovascular comorbidity in non-sleepy OSA [4]. Severity of nocturnal hypoxemia could be another explanation. Nocturnal intermittent hypoxia in the current ESADA cohort predicted prevalent hypertension [5] and impaired ventricular relaxation during diastole in another study [6]. Proportion of sleep time spent at an oxygen saturation below 90% was independently associated with an increased risk of hypertension [7] or a higher systolic blood pressure during both sleep and awake in OSA patients [8]. Further, in community-dwelling elderly with OSA, hypoxia was associated with insomnia only in those with CVD [9].
Prevalence estimations of physical and mental disease vary between countries and regions and may impact on comorbidity among OSA phenotypes. For instance, the age-standardized CVD prevalence rates are relatively high in Eastern and Central European countries and lower in Western, Northern, and Southern Europe [10]. Other disorders like chronic depression and insomnia also differ by region [11]. Finally, perception of OSA among health care providers and lay people will determine the characteristics of the patients referred for sleep studies. Therefore, referral patterns among geographical regions are likely to differ and may result in distinct distribution of clinical phenotypes of OSA.
The aim of the current study was to further investigate the concept of different OSA phenotypes. We hypothesized that the previously reported high occurrence of comorbidities in OSA patients with an insomnia phenotype at least in part is mediated by nocturnal hypoxia. Moreover, we speculated that the spectrum of the clinical OSA phenotypes differs between European geographical regions.
Methods
The European Sleep Apnea Database (ESADA) has prospectively collected data from unselected adult patients aged 18 to 80 years referred to several European sleep centers with a history of snoring and other symptoms suggesting OSA like witnessed apneas or increased daytime sleepiness [12]. Comorbidities like cardiovascular, pulmonary, metabolic, and psychiatric diseases based on medical records were also reported to the ESADA database. The study protocol was reviewed and approved by a local ethics committee at each participating center. All patients gave their written, informed consent. Patient data were coded and de-linked before entry into the central database. Data recorded between March 2007 and May 2016 were submitted for analyses. The cohort comprised 19,556 adult patients of which 17,325 (29.9% females) had complete data. The cohort was divided into five subcohorts according to geographical region (North, East, South, West, and Central) (Fig. 1).
The influence of region on clinical patient characteristics (phenotype) was studied after adjustment for age, gender, and BMI. Four clinical phenotypes were defined according to daytime symptoms (EDS) and characteristics suggestive of insomnia (self-reported sleep duration, sleep latency, diagnosed insomnia, or hypnotic use defined by ATC code N05) as reported previously [1]. ATC code N05 includes antipsychotics, anxiolytics, hypnotics, and sedatives. In brief, the criteria for the subgroups were as follows: (1) EDS (daytime+/nighttime−), (2) EDS/insomnia (daytime+/nighttime+), (3) non-EDS/non-insomnia (daytime−/nighttime−), and (4) insomnia (daytime−/nighttime+). Daytime+ indicates that the patient had daytime sleepiness defined by ESS score > 10 and daytime− that ESS score was ≤ 10. Criteria for nighttime+ included at least one of the following: diagnosis of insomnia, self-reported sleep latency ≥ 30 min, self-reported sleep duration ≤ 6 h, or use of hypnotics defined by ATC code N05. Nighttime− referred to situation where none of the nighttime+ criteria were fulfilled. The scoring methods used for polysomnography (PSG) or level 3 cardiorespiratory polygraphy (PG) have been reported previously [1, 12, 13]. A survey was made among participating centers to evaluate possible differences in standard operating procedures (SOPS) of handling referrals or other factors influencing referral patterns. The survey included four questions: possible mandatory screening of sleepiness before referral, categorical reasons for denying CPAP treatment or referrals to sleep studies and finally, which issues may impact on referral patterns for a sleep study.
Statistical methods
Data are presented as mean ± standard deviation or as frequency (%). Comparisons among the groups or phenotypes were performed using ANOVA for continuous variables, or the chi-square tests for categorical variables. Impact of age, gender, body mass index (BMI), current smoking, nocturnal hypoxia (mean oxygen saturation (SaO2)), minimum SaO2, oxygen desaturation index (ODI of 4%), apnea hypopnea index (AHI), and geographical region on prevalence of cardiovascular diseases among clinical presentation phenotypes was analyzed by logistic regression. Statistical analyses were performed using IBM SPSS Statistics 22.0 (Armonk, NY, USA: IBM Corp.). P value < 0.05 was considered statistically significant. All tests were two-sided.
Results
Anthropometric measures, comorbidities, and sleep apnea activity in relation to region and clinical phenotypes
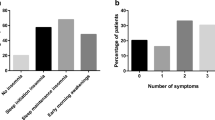
Regional differences were clinically relevant for age (minimum 48 vs. maximum 54 years), BMI (29 vs. 34 kg/m2), and ODI (15 vs. 32/h) (Table 1, Online Resource 1). The highest proportion of females was reported in the North region. The youngest, but most obese and sleepy patients, were found in the West, the most severe OSA in the South and the mildest degree of the disorder in the North region. In addition, the prevalence of the four defined clinical phenotypes varied between the European regions (Table 2, Fig. 2). The insomnia phenotype (alone or together with EDS) was the dominant phenotype in all regions. Isolated insomnia, however, was less common in the West.
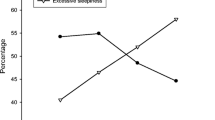
Clinical phenotypes differed in terms of comorbidity profile. Sleep apnea appeared to be more severe in the EDS group but less severe among those characterized with insomnia (P < 0.001). Conversely, cardiovascular morbidity was most prevalent among those with insomnia. A metabolic condition and/or a pulmonary disorder was more common in those with EDS combined with insomnia while the highest prevalence of a psychiatric disorder was found in those with insomnia or insomnia with EDS (Table 3, Fig. 3, Online Resource 2). Insomnia and EDS-insomnia phenotypes were more prevalent among women than men (Table 4).
SOPS of handling referrals did not explain regional differences in clinical phenotypes. Seven sites (two in the South, two in the East, two in the Central, and one in the West region) required screening of sleepiness prior sleep study. None of the sites denied CPAP treatment categorically for patients with specific comorbidities. Knowledge of referring physicians, long waiting lists, and public awareness campaigns regarding sleep in media were reported to influence referral patterns in all regions.
Predictors of cardiovascular disease in different phenotypes
When measures of nocturnal hypoxemia, age, gender, BMI, smoking, and geographical region were entered in a logistic regression analysis, higher prevalence of CVD was independently associated with lower nadir SaO2 in the EDS-insomnia and insomnia phenotypes (Table 5). For example, in EDS-insomnia phenotype, a 1% decrease in nadir SaO2 increases the risk of CVD with 1.6%. Accordingly, a 5% lower nadir saturation increases the risk for comorbid CVD by 10.5% ((1.6%)^5). When adding AHI instead of ODI in the logistic regression model, the results remained unchanged (data not shown). Unfortunately, the data on time spent below oxygen saturation of 90% (T < 90%) was only available in a subgroup of 7742 patients (44.6% of total). In this subgroup, T < 90% explained significantly CVD in univariate analysis in all phenotypes but not after adjustments (data not shown). Additional independent risk factors for CVD included age and BMI among all phenotypes, male gender in all groups except the non-EDS non-insomnia type, and current smoking in the EDS phenotype (Table 5). In terms of geographical regions, the East region had the highest risk for CVD across all phenotypes whereas the West was associated with less prevalent CVD.
Discussion
The present study provides three major findings in patients referred for suspected OSA to European sleep clinics. First, insomnia-like symptoms appeared to dominate the clinical presentation. Second, measures of nocturnal hypoxemia were independently associated with CVD comorbidity in phenotypes with insomnia-like symptoms. Third, single clinical characteristics and clinical OSA phenotypes differed among European geographical regions. However, the burden of comorbidities was high across all geographical regions and clinical phenotypes.
Association between phenotypes and comorbidities
Although the presence of comorbid insomnia [14, 15] has been recognized in OSA, the endeavor of phenotyping OSA is quite recent [1, 16,17,18]. The clinical characteristics (ESS score, subjective sleep duration and sleep latency, physician-diagnosed sleep disorder, use of hypnotics) that were applied to phenotype patients in the present study are readily available to clinicians treating patients with OSA. Insomnia-like phenotypes (EDS-insomnia and insomnia) were identified in more than 50% of patients, thereby confirming previous findings [1, 19]. Cardiovascular, pulmonary, and psychiatric comorbidities were more prevalent in phenotypes with insomnia-like symptoms. These results are in line with the findings in an Icelandic OSA cohort using quite similar phenotyping criteria [18] and our previous report [1].
Traditional risk factors such as age, gender, BMI, or smoking explained partly the comorbidity burden among distinct clinical phenotypes in our study. It has been suggested that an increased time lag from the start of the disease to final diagnosis and treatment in these OSA patients with rather atypical symptoms allowed for a higher exposure to harmful OSA-induced cardiovascular consequences [18]. Unfortunately, our database does not allow an assessment of the duration from the first OSA symptoms to diagnosis.
The higher prevalence of cardiovascular comorbidity in phenotypes with insomnia-like symptoms could also be explained by elevated adrenergically mediated alertness manifested as long sleep latency or short self-reported sleep duration. This “hyperarousal”-hypothesis is supported by observations of higher sympathetic activity in non-sleepy patients with severe OSA compared with the sleepy ones [3] as well as in primary insomniacs compared with good sleepers [20]. In fact, previous data has linked cardiovascular comorbidity to non-sleepy OSA in patients with peripheral arterial disease [4]. Epidemiological studies have also confirmed high cardiovascular comorbidity in depressive disorders [21], a condition with frequent symptoms of insomnia. Our data are also in concordance with the literature suggesting that the EDS plus insomnia group is more obese. Insomnia is a potential factor behind weight gain, an observation that has been explained by altered leptin and ghrelin activity and higher glucose and insulin levels as well as an increased appetite [22].
An important novel finding of our study was that nocturnal hypoxia, particularly nadir SaO2, was independently associated with higher prevalence of CVD in both phenotypes with insomnia-like symptoms. In the current analysis, three measures of hypoxia were included (ODI, mean, and lowest saturation) in the model and this modifies the statistical power of the single variable AHI or ODI. In fact, when taken as single OSA variable in the analysis, ODI increases the risk for CVD in all phenotype classes. However, it is important to highlight that the nadir of nocturnal hypoxia, a value which is not often in the focus of clinical sleep medicine, was a predictor of CV disease in the insomnia phenotypes. Indeed, recent studies demonstrated that subjects with chronic insomnia have increased sympathetic activity, an impaired sympathetic baroreflex function, and an augmented neural cardiovascular responsiveness to stress [23]. Further, in a Swedish study of community-dwelling elderly subjects with OSA, there was an association between spending more than 1.5% of the sleep time with at a SaO2 < 90% and insomnia in those with a CVD [9]. In summary, our data point towards a potential cumulative risk of insomnia and hypoxemia in OSA patients.
Geographical differences
Our study is the first to address geographical differences in the distribution of the clinical presentation phenotypes in European OSA patients. One of our key findings was that single clinical characteristics and the proportions of distinct clinical presentation phenotypes in the ESADA cohort differed across geographical regions. The finding that insomnia phenotypes are less frequent in Western and Central Europe is in concordance with the report of Dregan and coworkers [24]. In middle-aged and elderly population, they found that insomnia was less common in the western and central part of Europe at least with regard to Ireland and Germany. In the UK, insomnia was similar to Belgium, France, and Bulgaria but higher than in Germany or in northern countries.
The prevalence of comorbidities including cardiovascular disease [25, 26], metabolic syndrome [27], mood disorders [28], and obstructive lung disease [29] was, as expected, high in the ESADA cohort. CVD was most prevalent in the East, metabolic comorbidity in the Central region, and pulmonary and psychiatric comorbidity in the West region, the associations with region and comorbidity being in some cases quite high. The observed differences may at least in part reflect geographical differences in comorbidity according to EU statistics [10, 30, 31]. Also, lifestyle factors such as smoking or physical activity may explain differences [30]. Patients with chronic depression frequently suffer from insomnia and the proportion of the population reporting chronic depression is higher in the northern, western, and central Europe compared to southern and eastern Europe [31]. Particularly high prevalence rates of insomnia have been reported in Poland, Hungary, Estonia, Germany, France, and Portugal but much lower rates in Denmark, Italy, and the Netherlands [11]. In summary, data from this largest cohort or European sleep apnea patients point towards substantial geographical differences in comorbidity and clinical phenotype which may explain the heterogeneity of OSA management between countries. Our data also promote further research to gain deeper insight and to allow better interpretation of the regional effects and risk of comorbidities.
Referral routines
The management of OSA in Europe is variable [32] and cultural factors, general public, and medical awareness of OSA as well as available diagnostic facilities and treatment options might explain the observed differences. It is also likely that the perception of what constitutes a “typical OSA” patient varies considerably among health care providers in different regions. Although the prevalence rates of EDS and insomnia linked with distinct clinical phenotypes varied considerably, we need to acknowledge that cultural differences in attitudes to sleep may have an impact on how respondents in different countries interpret sleep problems and subsequently rate their sleep [33].
It might be argued that the differences seen in the clinical phenotypes between regions result from referral bias. However, thorough investigation of SOPS to handle referrals did not explain regional differences in clinical phenotypes. On the other hand, for example, media campaigns of awareness of sleep apnea were reported to influence referral patterns in all regions. Further population-based epidemiological studies in the different European regions may help to identify the role of referral bias as the underlying cause for the observed differences in our study.
Strengths and limitations
The ESADA cohort provides a unique opportunity to explore the real-life clinical practice and characteristics of OSA in different parts of Europe. Although, the ESADA does not reflect the individuals in the general population, they are part of a referral bias which in part may reflect the observed regional differences in clinical phenotypes in Europe. The wide age range and notable female representation in the cohort also allow a consideration of age and gender-related issues. The centralized data monitoring and web-based report format ensure uniformity in the reported data sets. In the ESADA protocol, apneas and hypopneas were significant respiratory events and RERA (respiratory effort related arousals) or RDI (respiratory disturbance index) were not scored. However, phenotypes were not defined based on sleep apnea data. Therefore, the conclusions in terms of regional distribution or comorbidity burden related to phenotypes are considered not to be affected by lack of RERA or RDI. The locally used diagnostic routines applied at participating centers provide a specific methodological challenge (for example, lack of comprehensive pulmonary function tests), which may contribute to differences in the reported comorbidities. A major limitation in our study was the broad definition of insomnia, which did not comply with the ICD or DSM criteria and therefore may lead to overestimation of the prevalence of “real” insomnia. Until now there is no special classification of insomnia with regard to severity but there are first ideas to cluster patients by polysomnography [34] or by the history of disease. However, the finding that even symptoms of insomnia in OSA patients are associated with increased comorbidity is of importance. Although the database does not allow for comprehensive analysis of the effects of sociodemographic factors (for example, socio-economic status, degree of physical exercise, marital status, or caffeine intake) on referral patterns or comorbidity of OSA, it represents the by far most comprehensive description of clinical characteristics in European OSA patients and has revealed a novel finding of link between nocturnal hypoxemia and cardiovascular comorbidity in OSA phenotypes with insomnia-like symptoms. Finally, our finding does not suggest a bias related to SOPs of handling referrals, since the finding was independent of geographical region.
Clinical implications
Interestingly, comorbid insomnia or insomnia-like symptoms may aggravate the burden of OSA with respect to cognitive function and vigilance. Identifying those patients has implications for personalized treatment. Insomnia-like symptoms have been associated with lower adherence to continuous positive airway pressure (CPAP) therapy [1, 2]. Moreover, treatment effects on outcomes like blood pressure, prevention of cardiovascular events, traffic accident rate, or mood disturbance are likely to differ depending on OSA phenotype. A recent study has demonstrated that cognitive behavioral therapy (CBTi) is effective also in patients with comorbid insomnia and OSA [35]. Combining CBTi with CPAP treatment might improve adherence to CPAP therapy and improve morning restfulness and daytime alertness in patients with OSA [14] and possibly protect from CVD events.
Conclusions
High prevalence of particularly cardiovascular comorbidity among patients with insomnia-like symptoms was linked to nocturnal hypoxemia. Characteristics of patients referred for suspected OSA differed between European sleep centers independently of confounders like age, gender, and obesity. Considerable differences in clinical presentation were found among OSA patients across Europe. In consideration of the wide generalized spread of patients in the Pan-European database, the ESADA database may turn to be particularly useful for the analysis on how clinical phenotypes may influence treatment outcomes in OSA.
References
Saaresranta T, Hedner J, Bonsignore MR, Riha RL, McNicholas WT, Penzel T, Anttalainen U, Kvamme JA, Pretl M, Sliwinski P, Verbraecken J, Grote L, ESADA Study Group (2016) Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS One 11(10):e0163439
Keenan BT, Kim J, Singh B, Bittencourt L, Chen NH, Cistulli PA, Magalang UJ, McArdle N, Mindel JW, Benediktsdottir B, Arnardottir ES, Prochnow LK, Penzel T, Sanner B, Schwab RJ, Shin C, Sutherland K, Tufik S, Maislin G, Gislason T, Pack AI (2018) Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep 1(41):3
Taranto Montemurro L, Floras JS, Picton P, Kasai T, Alshaer H, Gabriel JM, Bradley TD (2014) Relationship of heart rate variability to sleepiness in patients with obstructive sleep apnea with and without heart failure. J Clin Sleep Med 10(3):271–276
Utriainen KT, Airaksinen JK, Polo O, Raitakari OT, Pietilä MJ, Scheinin H, Helenius HY, Leino KA, Kentala ES, Jalonen JR, Hakovirta H, Salo TM, Laitio TT (2013) Unrecognised obstructive sleep apnoea is common in severe peripheral arterial disease. Eur Respir J 41(3):616–620
Tkacova R, McNicholas WT, Javorsky M, Fietze I, Sliwinski P, Parati G, Grote L, Hedner J (2014) European Sleep Apnoea Database study collaborators. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J 44(4):931–941
Fung JW, Li TS, Choy DK, Yip GW, Ko FW, Sanderson JE, Hui DS (2002) Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest 121(2):422–429
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283(14):1829–1836
Xu J, Ding N, Zhang X, Wang N, Sun B, Zhang R, Xie X, Wan Z, Gu Y, Zhang S, Hong Y, Huang M, Meng Z (2018) Nocturnal blood pressure fluctuation and associated influential factors in severe obstructive sleep apnea patients with hypertension. Sleep Breath. https://doi.org/10.1007/s11325-018-1634-6
Johansson P, Svensson E, Alehagen U, Jaarsma T, Broström A (2015) The contribution of hypoxia to the association between sleep apnoea, insomnia, and cardiovascular mortality in community-dwelling elderly with and without cardiovascular disease. Eur J Cardiovasc Nurs 14(3):222–231
European Cardiovascular Disease Statistics 2017. www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html. Last accessed 29 June 2018
van de Straat V, Bracke P (2015) How well does Europe sleep? A cross-national study of sleep problems in European older adults. Int J Public Health 60(6):643–650
Hedner J, Grote L, Bonsignore M, McNicholas W, Lavie P, Parati G, Sliwinski P, Barbé F, De Backer W, Escourrou P, Fietze I, Kvamme JA, Lombardi C, Marrone O, Masa JF, Montserrat JM, Penzel T, Pretl M, Riha R, Rodenstein D, Saaresranta T, Schulz R, Tkacova R, Varoneckas G, Vitols A, Vrints H, Zielinski J (2011) The European Sleep Apnoea Database (ESADA): report from 22 European sleep laboratories. Eur Respir J 38(3):635–642
Escourrou P, Grote L, Penzel T, Mcnicholas WT, Verbraecken J, Tkacova R, Riha RL, Hedner J, ESADA Study Group (2015) The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res 24(6):730–738
Smith S, Sullivan K, Hopkins W, Douglas J (2004) Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 5(5):449–456
Björnsdóttir E, Janson C, Sigurdsson JF, Gehrman P, Perlis M, Juliusson S, Arnardottir ES, Kuna ST, Pack AI, Gislason T, Benediktsdóttir B (2013) Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep 36(12):1901–1909
Lee RW, Sutherland K, Chan AS, Zeng B, Grunstein RR, Darendeliler MA, Schwab RJ, Cistulli PA (2010) Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep 33(9):1249–1254
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188(8):996–1004
Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, Benediktsdottir B, Gislason T (2014) The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 44(6):1600–1607
Bonsignore MR, Suarez Giron MC, Marrone O, Castrogiovanni A, Montserrat JM (2017) Personalised medicine in sleep respiratory disorders: focus on obstructive sleep apnoea diagnosis and treatment. Eur Respir Rev 26(146):170069
de Zambotti M, Cellini N, Baker FC, Colrain IM, Sarlo M, Stegagno L (2014) Nocturnal cardiac autonomic profile in young primary insomniacs and good sleepers. Int J Psychophysiol 93(3):332–339
Halaris A (2013) Inflammation, heart disease, and depression. Curr Psychiatry Rep 15(10):400
Schmid SM, Hallschmid M, Schultes B (2015) The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 3(1):52–62 Review. Erratum in: Lancet Diabetes Endocrinol 2014 May;2(5):e12
Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Cauter EV (2018) Assessment of sympathetic neural activity in chronic insomnia: evidence for elevated cardiovascular risk. Sleep 41(6)
Dregan A, Armstrong D (2011) Cross-country variation in sleep disturbance among working and older age groups: an analysis based on the European Social Survey. Int Psychogeriatr 23(9):1413–1420
Peker Y, Carlson J, Hedner J (2006) Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 28(3):596–602
McNicholas WT, Bonsigore MR, Management Committee of EU COST ACTION B26 (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29(1):156–178
Bonsignore MR, Esquinas C, Barceló A, Sanchez-de-la-Torre M, Paternó A, Duran-Cantolla J, Marín JM, Barbé F (2012) Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J 39(5):1136–1143
Saunamäki T, Jehkonen M (2007) A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand 115(1):1–11
Anttalainen U, Polo O, Vahlberg T, Saaresranta T (2010) Reimbursed drugs in patients with sleep-disordered breathing: a static-charge-sensitive bed study. Sleep Med 11(1):49–55
European Core Health Indicators (ECHI). https://ec.europa.eu/health/indicators_data/echi_en. Last accessed 29 June 2018
Eurostat. http://ec.europa.eu/eurostat. Last accessed 29 June 2018
Fietze I, Penzel T, Alonderis A, Barbe F, Bonsignore MR, Calverly P, De Backer W, Diefenbach K, Donic V, Eijsvogel MM, Franklin KA, Gislason T, Grote L, Hedner J, Jennum P, Lavie L, Lavie P, Levy P, Lombardi C, Mallin W, Marrone O, Montserrat JM, Papathanasiou ES, Parati G, Plywaczewski R, Pretl M, Riha RL, Rodenstein D, Saaresranta T, Schulz R, Sliwinski P, Steiropoulos P, Svaza J, Tomori Z, Tonnesen P, Varoneckas G, Verbraecken J, Vesely J, Vitols A, Zielinski J, McNicholas WT, COST Action B26 Group (2011) Management of obstructive sleep apnea in Europe. Sleep Med 12(2):190–197
Leger D, Poursain B (2005) An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin 21(11):1785–1789
Miller CB, Bartlett DJ, Mullins AE, Dodds KL, Gordon CJ, Kyle SD, Kim JW, D’Rozario AL, Lee RS, Comas M, Marshall NS, Yee BJ, Espie CA, Grunstein RR (2016) Clusters of insomnia disorder: an exploratory cluster analysis of objective sleep parameters reveals differences in neurocognitive functioning, quantitative EEG, and heart rate variability. Sleep 39(11):1993–2004
Sweetman A, Lack L, Lambert S, Gradisar M, Harris J (2017) Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med 39:38–46
Acknowledgements
Open access funding provided by University of Turku (UTU) including Turku University Central Hospital. Excellent technical assistance in terms of center monitoring and database maintenance was provided by Ann-Christin Lundquist, RN, and Jeanette Norum, RN.
Funding
The ESADA network has received support from the European Union COST action B26. The European Respiratory Society (ERS) supports the ESADA for the second period as a Clinical Research Collaboration (CRC) (2015–2017 and 2018–2020). Unrestricted seeding grants from ResMed Inc. and Philips Respironics Inc. for establishment of the organization and the database are gratefully acknowledged.
Collaborators in the ESADA project (Alphabetical order):
Anttalainen U, Division of Medicine, Department of Pulmonary Diseases, Turku University Hospital and Sleep Research Centre, Department of Physiology, University of Turku, Finland
Barbé F, Servei Pneumologia Hospital Arnau de Vilanova and Hospital Santa Maria, Lleida, and CIBERes, Madrid, Spain
Bonsignore MR, Biomedical Department of Internal and Specialistic Medicine (DiBiMIS), University of Palermo; and CNR Institute of Biomedicine and Molecular Immunology, Palermo, Italy
Basoglu O, Department of Chest Diseases, Ege University, Izmir, Turkey
Bielicki P, Department of Internal Medicine, Pneumonology and Allergology, Warsaw Medical University, Warsaw, Poland
Bouloukaki I. Sleep Disorders Unit, Department of Respiratory Medicine, Medical School, University of Crete, Greece
Dogas Z, Split Sleep Medicine Center and Department of Neuroscience, University of Split School of Medicine, Split, Croatia
Dorkova Z, Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium
Escourrou P, Service d’Éxplorations Fonctionnelles Multidisciplinaires, Hospital Antoine-Beclere, Clamart, France
Fietze I, Center of Sleep Medicine, Charité – Universitätsmedizin Berlin, Germany
Esquinas C, Servei de Pneumologia, Hospital Universitari Arnau de Vilanova, Lleida, Spain
Grote L, Department of Sleep Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden
Hayes L, Pulmonary and Sleep Disorders Unit, St. Vincent’s University Hospital, Dublin, Ireland
Hedner J, Department of Sleep Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden
Joppa P, Department of Respiratory Medicine and Tuberculosis, Faculty of Medicine, P.J.Safarik University and L. Pasteur University Hospital, Kosice, Slovakia
Kurki S, Auria Biobank, University of Turku and Turku University Hospital, Turku, Finland
Kvamme JA, Department of ENT, Førde Central Hospital, Førde, Norway
Tamisier R, Université Grenoble Alpes, INSERM HP2 (U1042) and Grenoble University Hospital, Grenoble, France
Lombardi C, Istituto Auxologico Italiano, Ospedale San Luca, Milan, Italy
Marrone O, CNR Institute of Biomedicine and Molecular Immunology, Palermo, Italy
Masa JF, Hospital San Pedro de Alcàntara, Cáceres, Spain
McNicholas WT, Department of Respiratory and Sleep Medicine, St. Vincent’s University Hospital, Dublin, and Conway Research Institute, University College Dublin, Ireland
Montserrat JM, Hospital Clinic i Provincial de Barcelona, Barcelona, IDIBAPS Barcelona and CIBERes, Madrid, Spain
Parati G, Istituto Auxologico Italiano, Ospedale San Luca, Milan, Italy
Pataka A, Respiratory Failure Unit, G. Papanikolaou Hospital, Thessalonika, Greece
Penzel T, Schlafmedizinisches Zentrum, Charité–Universitätsmedizin Berlin, Germany and International Clinical Research Center, St. Anne’s University Hospital Brno, Brno, Czech Republic
Pépin JL, Université Grenoble Alpes, INSERM HP2 (U1042) and Grenoble University Hospital, Grenoble, France
Pretl M, Centre for Sleep and Waking Disorders, Department of Neurology, First Faculty of Medicine, Charles University, Prague, and Inspamed, Neurology and Sleep Laboratory, Prague, Czech Republic
Riha RL, Department of Sleep Medicine, Royal Infirmary of Edinburgh, United Kingdom
Roisman G, Unité de Médecine du Sommeil, Hopital Antoine-Beclere, Clamart, France
Ryan S, Department of Respiratory and Sleep Medicine, St. Vincent’s University Hospital, Dublin, Conway Research Institute, University College Dublin, Ireland
Saaresranta T, Division of Medicine, Department of Pulmonary Diseases, Turku University Hospital; and Sleep Research Centre, Department of Physiology, University of Turku, Turku, Finland
Schiza SE. Sleep Disorders Unit, Department of Respiratory Medicine, Medical School, University of Crete, Greece
Schulz R, Sleep Disorders Centre, University of Giessen, Lung Centre, Giessen, Germany
Sliwinski P, Institute of Tuberculosis and Lung Diseases, 4th Department of Respiratory Medicine, Warsaw, Poland
Staats R, Department of Respiratory Medicine, Hospital de Santa Maria, Lisbon, Portugal
Steiropoulos P, Sleep Unit, Department of Pneumonology, Democritus University of Thrace, Alexandroupolis, Greece
Tasbakan MS, Department of Chest Diseases, Ege University, Izmir, Turkey
Tkacova R, Department of Respiratory Medicine and Tuberculosis, Faculty of Medicine, P.J. Safarik University and L. Pasteur University Hospital, Kosice, Slovakia
Varoneckas G, Institute Psychophysiology and Rehabilitation, Palanga, Lithuania
Verbraecken J, Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium
Vrints H, Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Anttalainen, U., Grote, L., Fietze, I. et al. Insomnia symptoms combined with nocturnal hypoxia associate with cardiovascular comorbidity in the European sleep apnea cohort (ESADA). Sleep Breath 23, 805–814 (2019). https://doi.org/10.1007/s11325-018-1757-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1757-9