Abstract
Purpose
The BACE inhibitor verubecestat was previously found to reduce amyloid load as assessed by 18F-flutemetamol positron emission tomography (PET) composite cortical standard uptake value ratio (SUVr) in patients with mild-to-moderate Alzheimer’s disease (AD) in a substudy of the EPOCH trial. Here, we report on additional analyses relevant to the EPOCH PET data, to help inform on the use of PET for assessing amlyloid load in AD clinical trials.
Procedures
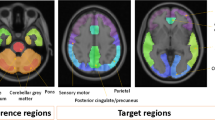
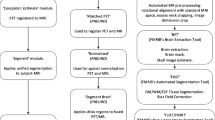
The analyses addressed (1) identification of an optimal 18F-flutemetamol reference region, (2) determination of the threshold to characterize the magnitude of the longitudinal change, and (3) the impact of partial volume correction (PVC). Pons and subcortical white matter were evaluated as reference regions. The SUVr cutoffs and final reference region choice were determined using 162 18F-flutemetamol PET scans from the AIBL dataset. 18F-flutemetamol SUVrs were computed at baseline and at Week 78 in EPOCH participants who received verubecestat 12 mg (n = 14), 40 mg (n = 20), or placebo (n = 20). Drug effects on amyloid load were computed using either Meltzer (MZ), or symmetric geometric transfer matrix (SGTM) PVC and compared to uncorrected data.
Results
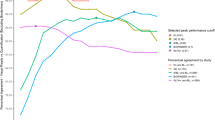
The optimal subcortical white matter and pons SUVr cutoffs were determined to be 0.69 and 0.62, respectively. The effect size to detect longitudinal change was higher for subcortical white matter (1.20) than pons (0.45). Hence, subcortical white matter was used as the reference region for the EPOCH PET substudy. In EPOCH, uncorrected baseline SUVr values correlated strongly with MZ PVC (r2 = 0.94) and SGTM PVC (r2 = 0.92) baseline SUVr values, and PVC did not provide improvement for evaluating treatment effects on amyloid load at Week 78. No change from baseline was observed in the placebo group at Week 78, whereas a 0.02 and a 0.04 decrease in SUVr were observed in the 12 mg and 40 mg arms, with the latter representing a 22% reduction in the amyloid load above the detection threshold.
Conclusions
Treatment-related 18F-flutemetamol longitudinal changes in AD clinical trials can be quantified using a subcortical white matter reference region without PVC.
Clinical trial registration: clinicaltrials.gov NCT01739348.




Similar content being viewed by others
Abbreviations
- AAL:
-
Automated Anatomical Labeling
- AD:
-
Alzheimer’s disease
- AIBL:
-
Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing
- APP:
-
Amyloid precursor protein
- AUC:
-
Area under the curve
- BACE:
-
β-Site amyloid precursor protein cleaving enzyme
- CSF:
-
Cerebrospinal fluid
- CI:
-
Confidence interval
- LS:
-
Least squares
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- MZ:
-
Meltzer
- PET:
-
Positron emission tomography
- PiB:
-
Pittsburgh Compound B
- PVC:
-
Partial volume correction
- ROC:
-
Receiver operating characteristics
- SD:
-
Standard deviation
- SGTM:
-
Symmetric geometric transfer matrix
- SPM12:
-
Statistical Parametric Mapping 12
- SUVr:
-
Standard uptake value ratio
References
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 68:1–14
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608
Femminella GD, Thayanandan T, Calsolaro V et al (2018) Imaging and molecular mechanisms of Alzheimer’s disease: a review. Int J Mol Sci 19:3702
Vandenberghe R, Adamczuk K, Dupont P, Laere KV, Chételat G (2013) Amyloid PET in clinical practice: Its place in the multidimensional space of Alzheimer’s disease. Neuroimage Clin 2:497–511
Klunk WE, Engler H, Nordberg A et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55:306–319
Nelissen N, Van Laere K, Thurfjell L et al (2009) Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med 50:1251–1259
Vandenberghe R, Van Laere K, Ivanoiu A et al (2010) 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 68:319–329
Adamczuk K, Schaeverbeke J, Nelissen N et al (2016) Amyloid imaging in cognitively normal older adults: comparison between (18)F-flutemetamol and (11)C-Pittsburgh compound B. Eur J Nucl Med Mol Imaging 43:142–151
Mountz JM, Laymon CM, Cohen AD et al (2015) Comparison of qualitative and quantitative imaging characteristics of [11C]PiB and [18F]flutemetamol in normal control and Alzheimer’s subjects. Neuroimage Clin 9:592–598
Thurfjell L, Lilja J, Lundqvist R et al (2014) Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 55:1623–1628
Duff K, Horn KP, Hoffman JM (2019) Long-term changes in 18F-flutemetamol uptake in nondemented older adults. Alzheimer Dis Assoc Disord 33:113–117
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thal DR, Beach TG, Zanette M et al (2018) Estimation of amyloid distribution by [18F]flutemetamol PET predicts the neuropathological phase of amyloid β-protein deposition. Acta Neuropathol 136:557–567
Bucci M, Savitcheva I, Farrar G et al (2021) A multisite analysis of the concordance between visual image interpretation and quantitative analysis of [(18)F]flutemetamol amyloid PET images. Eur J Nucl Med Mol Imaging 48:2183–2199
Chen K, Roontiva A, Thiyyagura P et al (2015) Improved power for characterizing longitudinal amyloid-beta PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med 56:560–566
Schwarz CG, Gunter JL, Lowe VJ et al (2019) A comparison of partial volume correction techniques for measuring change in serial amyloid PET SUVR. J Alzheimers Dis 67:181–195
Yang J, Hu C, Guo N et al (2017) Partial volume correction for PET quantification and its impact on brain network in Alzheimer’s disease. Sci Rep 7:13035
Meltzer CC, Kinahan PE, Greer PJ et al (1999) Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med 40:2053–2065
Rousset OG, Ma Y, Evans AC (1998) Correction for partial volume effects in PET: principle and validation. J Nucl Med 39:904–911
Labbé C, Koepp M, Ashburner J, Spinks T, Richardson M, Duncan J, Cunningham V (1998) Absolute PET Quantification with Correction for Partial Volume Effects with Cerebral Structures. In: Carson RE, Daube-Witherspoon ME, Herscovitch P (eds) Quantitative functional brain imaging with positron emission tomography. Academic Press, San Diego, pp 59–66
Sattarivand M, Kusano M, Poon I, Caldwell C (2012) Symmetric geometric transfer matrix partial volume correction for PET imaging: principle, validation and robustness. Phys Med Biol 57:7101–7116
Thompson PM, Hayashi KM, de Zubicaray G et al (2003) Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci 23:994–1005
Blinkouskaya Y, Weickenmeier J (2021) Brain Shape Changes Associated with Cerebral Atrophy in Healthy Aging and Alzheimer's Disease. Front Mech Eng. https://doi.org/10.3389/fmech.2021.705653
Scott JD, Li SW, Brunskill AP et al (2016) Discovery of the 3-imino-1,2,4-thiadiazinane 1,1-dioxide derivative verubecestat (MK-8931)-a β-site amyloid precursor protein cleaving enzyme 1 inhibitor for the treatment of Alzheimer’s Disease. J Med Chem 59:10435–10450
Kennedy ME, Stamford AW, Chen X et al (2016) The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer's disease patients. Sci Transl Med 8:363ra150
Forman M, Palcza J, Tseng J et al (2019) Safety, tolerability, and pharmacokinetics of the beta-site amyloid precursor protein-cleaving enzyme 1 inhibitor verubecestat (MK-8931) in healthy elderly male and female subjects. Clin Transl Sci 12:545–555
Min KC, Dockendorf MF, Palcza J et al (2019) Pharmacokinetics and pharmacodynamics of the BACE1 inhibitor verubecestat (MK-8931) in healthy Japanese adults: a randomized, placebo-controlled study. Clin Pharmacol Ther 105:1234–1243
Vassar R (2014) BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res Ther 6:89
Vassar R, Bennett BD, Babu-Khan S et al (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741
Egan MF, Kost J, Tariot PN et al (2018) Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703
Sur C, Kost J, Scott D et al (2020) BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer’s disease brain. Brain 143:3816–3826
Landau SM, Thomas BA, Thurfjell L, the Alzheimer’s Disease Neuroimaging Intiative, et al (2014) Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging 41:398–1407
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Greve DN, Svarer C, Fisher PM et al (2014) Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage 92:225–236
Greve DN, Salat DH, Bowen SL et al (2016) Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage 132:334–343
Kalheim LF, Fladby T, Coello C, Bjørnerud A, Selnes P (2018) [18F]-Flutemetamol uptake in cortex and white matter: comparison with cerebrospinal fluid biomarkers and [18F]-fludeoxyglucose. J Alzheimers Dis 62:1595–1607
Heurling K, Buckley C, Vandenberghe R, Laere KV, Lubberink M (2015) Separation of β-amyloid binding and white matter uptake of (18)F-flutemetamol using spectral analysis. Am J Nucl Med Mol Imaging 5:515–526
Lowe VJ, Lundt E, Knopman D et al (2017) Comparison of [18F]flutemetamol and [11C]Pittsburgh Compound-B in cognitively normal young, cognitively normal elderly, and Alzheimer’s disease dementia individuals. NeuroImage Clinical 16:295–302
Fleisher AS, Joshi AD, Sundell KL et al (2017) Use of white matter reference regions for detection of change in florbetapir positron emission tomography from completed phase 3 solanezumab trials. Alzheimers Dement 13:1117–1124
Chiao P, Bedell BJ, Avants B et al (2019) Impact of reference and target region selection on amyloid PET SUV ratios in the phase 1b PRIME study of aducanumab. J Nucl Med 60:100–106
Salloway S, Honigberg LA, Cho W et al (2018) Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimers Res Ther 10:96
Thomas BA, Erlandsson K, Modat M et al (2011) The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 38:1104–1119
Schwarz CG, Senjem ML, Gunter JL et al (2017) Optimizing PiB-PET SUVR change-over-time measurement by a large-scale analysis of longitudinal reliability, plausibility, separability, and correlation with MMSE. Neuroimage 144:113–127
Villemagne VL, Pike KE, Chételat G et al (2011) Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 69:181–192
Rinne JO, Brooks DJ, Rossor MN et al (2010) 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9:363–372
Mintun MA, Lo AC, Duggan Evans C et al (2021) Donanemab in Early Alzheimer’s Disease. N Engl J Med 384:1691–1704
Wolk DA, Sadowsky C, Safirstein B et al (2018) Use of flutemetamol F18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol 75:1114–1123
Hatashita S, Wakebe D, Kikuchi Y, Ichijo A (2019) Longitudinal assessment of amyloid-β deposition by [18F]-flutemetamol PET imaging compared with [11C]-PIB across the spectrum of Alzheimer’s disease. Front Aging Neurosci 11:251
Mattsson N, Palmqvist S, Stomrud E, Vogel J, Hansson O (2019) Staging β-amyloid pathology with amyloid positron emission tomography. JAMA Neurol 76:1319–1329
Schwarz CG, Gunter JL, Wiste HJ et al (2016) A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin 11:802–812
Klunk WE, Koeppe RA, Price JC et al (2015) The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 11(1–15):e1-4
Acknowledgements
The authors would like to thank the study participants and staff. Medical writing assistance, under the direction of the authors, was provided by Kirsty Muirhead, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Christopher Lines (MSD) assisted with writing and editing of the paper.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
CS, JK, BN, JS, and MFE contributed to the conception, design, and planning of the study.
DS, JS, and MFE acquired the data.
CS, KA, DS, JK, JS, MS, and MFE analyzed the data.
CS, KA, DS, JK, CB, GF, JS, IB, and MFE interpreted the results.
All authors critically reviewed the manuscript for important intellectual content, and provided final approval of the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interests
CS, JK, IB, and MFE are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, & may own stock and/or stock options in Merck and Co., Inc., Rahway, NJ, USA.
KA, DS, MS, and JS are employees of Clario, San Mateo, CA, USA.
CB, GF, and BN are employees of GE Healthcare, Amersham, UK.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sur, C., Adamczuk, K., Scott, D. et al. Evaluation of 18F-flutemetamol amyloid PET image analysis parameters on the effect of verubecestat on brain amlyoid load in Alzheimer’s disease. Mol Imaging Biol 24, 862–873 (2022). https://doi.org/10.1007/s11307-022-01735-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01735-z