Abstract
Purpose
Early and precise localization of recurrent prostate cancer lesions after local therapy facilitates optimal disease management. Here, we present results from a single-center study to evaluate the utility of [18F]fluciclovine PET/CT to localize prostate cancer recurrence in patients with PSA <1 ng/mL.
Procedures
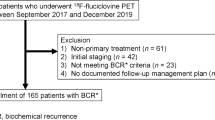
Data from men who underwent [18F]fluciclovine PET/CT (August 2016–March 2020) for suspected recurrent prostate cancer and who had a PSA value <1ng/mL were retrospectively reviewed. The number of positive scans (positivity rates, PR) was calculated for the whole body, prostate/bed, and extraprostatic regions (pelvic or extrapelvic lymph nodes, bones, and soft tissue). PR were stratified by pre-scan PSA.
Results
Data from 113 patients were included. In total, 98 (87%) were post-prostatectomy and 15 (13%) had received non-surgical primary therapy. Twenty patients (18%) were receiving ADT at the time of the scan, 91 (81%) were not, and ADT status was not known for 2 (1.8%) patients. The overall PR at PSA <1ng/mL was 59% (67/113). For the prostate/bed, it was 35% (40/113), and for extraprostatic locations, it was 37% (42/113). At PSA >0–<0.2, 0.2–<0.5, and 0.5–<1 ng/mL, the overall PR was 43% (10/23), 70% (35/50), and 55% (22/40), respectively. In the prostate/bed, these were 13% (3/23), 50% (25/50), and 30% (12/40), respectively, and in extraprostatic lesions were 30% (7/23), 44% (22/50), and 33% (13/40), respectively. Pelvic lymph nodes were the most common site for extraprostatic lesions (29/113, 26%). PR in extrapelvic lymph nodes, bone, and soft tissue were 8.0%, 12%, and 3.5%, respectively. Soft tissue lesions comprised lung nodules (n=3) and a perirectal mass implant (n=1).
Conclusions
Despite low PSA values, more than half of patients had positive [18F]fluciclovine PET/CT findings. Patients with low PSA levels may demonstrate suspicious findings outside of the pelvis, including abdominal lymph nodes and metastatic disease to bones and lungs.





Similar content being viewed by others
References
Mottet N, Bellmunt J, Briers E, et al. The EAU prostate cancer guidelines. 2020. http://uroweb.org/guideline/prostate-cancer. Accessed August 2021.
Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. 2020. https://gco.iarc.fr/today. Accessed August 2021.
Stephenson AJ, Scardino PT, Kattan MW et al (2007) Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 25:2035–2041
Tendulkar RD, Agrawal S, Gao T et al (2016) Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 34:3648–3654
King CR (2012) The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 84:104–111
Vargas HA, Martin-Malburet AG et al (2016) Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1ng/ml following prostatectomy: how much information can conventional imaging provide? Urol Oncol 34(482):e5–e10
Malone S, Croke J, Roustan-Delatour N et al (2012) Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. IJROBP 84:725–732
Jani AB, Schreibmann E, Goyal S et al (2021) 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. The Lancet 397:1895–904
Scarsbrook AF, Bottomley D, Teoh EJ et al (2020) Impact of 18F-fluciclovine positron emission tomography on the management of patients with recurrence of prostate cancer: Results from the FALCON trial. IJROBP 107:316–324
Schuster DM, Nieh PT, Jani AB et al (2014) Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 191:1446–1453
Andriole GL, Kostakoglu L, Chau A et al (2019) The impact of positron emission tomography with 18F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol 201:322–331
Pernthaler B, Kulnik R, Gstettner C et al (2019) A prospective head-to-head comparison of 18F-fluciclovine with 68Ga-PSMA-11 in biochemical recurrence of prostate cancer in PET/CT. Clin Nucl Med 44:e566–e573
Rais-Bahrami S, Efstathiou JA, Turnbull CM et al (2021) 18F-Fluciclovine PET/CT performance in biochemical recurrence of prostate cancer: a systematic review. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-021-00382-9
Tade FI, Sajdak RA, Gabriel M et al (2019) Best practices for 18F-fluciclovine PET/CT imaging of recurrent prostate cancer: a guide for technologists. J Nucl Med Tech 47:282–287
Nanni C, Zanoni L, Bach-Gansmo T et al (2020) [18F]Fluciclovine PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging-version 1.0. EJNMMI 47:579–591
Savir-Baruch B, Banks KP, McConathy JE et al (2018) ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med 43:909–917
Blue Earth Diagnostics. Axumin prescribing information at: https://www.axumin.com/prescribing-information.pdf. Accessed August 2021.
Pisansky TM, Thompson IM, Valicenti RK et al (2019) Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA guideline amendment 2018–2019. J Urol 202:533–538
Bach-Gansmo T, Nanni C, Nieh PT et al (2017) Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol 197:676–683
Dreyfuss AD, Ahn GS, Barsky AR et al (2021) 18F-Fluciclovine PET/CT in therapeutic decision making for prostate cancer: a large single-center practice-based analysis. Clin Nucl Med 46:187–194
Krohn T, Birmes A, Winz OH et al (2017) The reconstruction algorithm used for [68Ga]PSMA-HBED-CC PET/CT reconstruction significantly influences the number of detected lymph node metastases and coeliac ganglia. Eur J Nucl Med Mol Imaging 44(4):662–9
Teyateeti A, Teyateeti A, Ravizzini GC, et al (2021) Diagnostic performance of 18F-fluciclovine PET/CT in prostate cancer patients with rising PSA level ≤ 0.5 ng/ml after multiple treatment failures. Am J Nucl Med Mol Imaging 11(2):87–98
Teyateeti A, Teyateeti A, Macapinlac HA, Lu Y (2020) Is there any role for F-18 fluciclovine PET/CT in the presence of undetectable PSA in prostate cancer patients after definitive treatment? Clin Nucl Med 45:672–678
Malviya G, Patel R, Salji M et al (2020) 18F-Fluciclovine PET metabolic imaging reveals prostate cancer tumour heterogeneity associated with disease resistance to androgen deprivation therapy. EJNMMI Res 10:143
England JR, Paluch J, Ballas LK, Jadvar H (2019) 18F-Fluciclovine PET/CT detection of recurrent prostate carcinoma in patients with serum PSA ≤ 1 ng/mL after definitive primary treatment. Clin Nucl Med 44:e128–e132
Gandaglia G, Abdollah F, Schiffmann J et al (2014) Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate 74:210–216
Hess KR, Varadhachary GR, Taylor SH et al (2006) Metastatic patterns in adenocarcinoma. Cancer 106:1624–1633
Paño B, Sebastià C, Buñesch L et al (2011) Pathways of lymphatic spread in male urogenital pelvic malignancies. Radiographics 31:135–160
NCCN. NCCN clinical practice guidelines in oncology: prostate cancer. Version 4.2019. https://www2.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf 2019. Accessed August 2021.
Kim EH, Siegel BA, Teoh EJ, Andriole GL, LOCATE Study Group (2021) Prostate cancer recurrence in patients with negative or equivocal conventional imaging: a role for 18F-fluciclovine-PET/CT in delineating sites of recurrence and identifying patients with oligometastatic disease. Urol Oncol 39(365):e9–e16
Armstrong JM, Martin CR, Dechet C et al (2020) 18F-fluciclovine PET CT detection of biochemical recurrent prostate cancer at specific PSA thresholds after definitive treatment. Urol Oncol 38(636):e1–e6
Savir-Baruch B, Choyke PL, Rowe SP et al (2021) Role of 18F-fluciclovine and prostate-specific membrane antigen PET/CT in guiding management of oligometastatic prostate cancer: AJR expert panel narrative review. AJR 216:851–859
Acknowledgements
Medical writing support was provided by Dr Catriona Turnbull (BED).
Author information
Authors and Affiliations
Contributions
Study conception and design: BSB. Material preparation and data collection: JEB, PL, DG, BSB. All authors participated in data analysis and in drafting and reviewing the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
For this type of study, formal consent is not required.
Conflict of Interest
B. Savir-Baruch reports receiving research grants from Blue Earth Diagnostics. The authors have no further relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bulbul, J.E., Grybowski, D., Lovrec, P. et al. Positivity Rate of [18F]Fluciclovine PET/CT in Patients with Suspected Prostate Cancer Recurrence at PSA Levels Below 1 ng/mL. Mol Imaging Biol 24, 42–49 (2022). https://doi.org/10.1007/s11307-021-01644-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01644-7