Abstract
Purpose
Nanotheranostic platforms, i.e., the combination of both therapeutic and diagnostic agents on a single platform, are emerging as an interesting tool for the personalized cancer medicine. Therefore, the aim of this work was to evaluate the in vivo properties of a Tc-99m-labeled nanostructured lipid carrier (NLC) formulation, co-loaded with doxorubicin (DOX) and docosahexaenoic acid (DHA), for theranostic applications.
Procedures
NLC-DHA-DOX were prepared busing the hot melting homogenization method using an emulsification-ultrasound and were radiolabeled with Tc-99m. Biodistribution studies, scintigraphic images, and antitumor activity were performed in 4T1 tumor-bearing mice.
Results
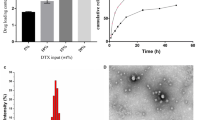
NCL was successfully radiolabeled with Tc-99m. Blood clearance showed a relatively long half-life, with blood levels decaying in a biphasic manner (T1/2 α = 38.7 min; T1/2 β = 516.5 min). The biodistribution profile and scintigraphic images showed higher tumor uptake compared to contralateral muscle in all time-points investigated. Antitumor activity studies showed a substantial tumor growth inhibition ratio for NLC-DHA-DOX formulation. In addition, the formulation showed more favorable toxicity profiles when compared to equivalent doses of free administered drugs, being able to reduce heart and liver damage.
Conclusions
Therefore, NLC-DHA-DOX formulation demonstrated feasibility in breast cancer treatment and diagnosis/monitoring, leading to a new possibility of a theranostic platform.






Similar content being viewed by others
References
Stewart BW, Wild CP (2014) World cancer report 2014 latest world cancer statistics. International Agency for Research on Cancer, Lyon
Livi L, Meattini I, de Cardillo CL et al (2009) Non-pegylated liposomal doxorubicin in combination with cyclophosphamide or docetaxel as first-line therapy in metastatic breast cancer: a retrospective analysis. Tumori 95:422–426
Shi Y, Moon M, Dawood S et al (2011) Mechanisms and management of doxorubicin cardiotoxicity. Herz 36:296–305
Primeau AJ, Rendon A, Hedley D et al (2005) The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res 27:8782–8788
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454
Carvalho C, Santos RX, Cardoso S et al (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16:3267–3285
Jassem J, Pieńkowski T, Płuzańska A et al (2001) Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol 19:1707–1715
Germain E, Chajès V, Cognault S et al (1998) Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: relationship to lipid peroxidation. Int J Cancer 75:578–583
Colas S, Mahéo K, Denis F et al (2006) Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: a role for tumor vascularization. Clin Cancer Res 12:5879–5886
Siddiqui RA, Harvey KA, Xu Z et al (2011) Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 37:399–412
Mahéo K, Vibet S, Steghens JP et al (2005) Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation. Free Radic Biol Med 39:742–751
Hardman WE, Avula CP, Fernandes G, Cameron IL (2001) Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin Cancer Res 7:2041–2049
Parhi P, Mohanty C, Sahoo SK (2012) Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 17:1044–1052
Mamot C, Drummond DC, Hong K et al (2003) Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Update 6:271–279
Muller RH, Mader K, Gohla D (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Mehnert W, Mader K (2001) Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–196
Janib SM, Moses AS, MacKay JA (2010) Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev 62:1052–1063
Jo SD, SH K, Won Y-Y et al (2016) Targeted nanotheranostics for future personalized medicine: recent progress in cancer therapy. Theranostics 6:1362–1377
Choi KY, Liu G, Lee S, Chen X (2012) Theranosticnanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nano 4:330–342
Xie J, Chen X (2010) Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 62:1064–1079
Yang DJ, Kim C, Schechter NR et al (2003) Imaging with 99mTc-ECDG targeted at the multifunctional glucose system: feasibility studies with rodents. Radiology 226:465–473
Saha GB (2010) Radiopharmaceuticals and methods of radiolabeling. In: Fundamentals of nuclear pharmacy. Springer, New York, pp 83–113
Mussi SV, Sawant R, Perche F et al (2014) Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm Res 8:1882–1890
Mussi SV, Silva RC, Oliveira MC et al (2013) New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur J Pharm Sci 48:282–290
Fernandes RS, Silva JO, Lopes SCA et al (2016) Technetium-99m-labeled doxorubicin as an imaging probe for murine breast tumor (4T1 cell line) identification. Nucl Med Commun 37:307–312
Thrall JH, Ziessman HA (2003) Medicina Nuclear, 2nd edn. Guanabara Koogan, Rio de Janeiro
Souza CM, Carvalho LF, Vieira TS et al (2012) Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed Pharmacother 66:491–498
Li Y, Wang J, Wientjes MG, JL A (2012) Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev 64:29–39
Maeda H, Wu J, Sawa J, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284
Torchilin V (2011) Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–135
Reddy LH, Sharma LK, Chuttani K et al (2005) Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release 105:185–198
Beloqui A, Solinis MA, Delgado A et al (2013) Biodistribution of nnostructured lipid carriers (NLCs) after intravenous administration to rats: influence of technological fators. Eur J Pharm Biopharm 84:309–314
Torchilin V (2009) Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm 71:431–444
Yuan F, Dellian M, Fukumura D et al (1995) Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 55:3752–3756
Leu AJ, Berk DA, Lymboussaki A et al (2000) Absence of functional lymphatics within murine sarcoma: molecular and functional evaluation. Cancer Res 60:4324–4327
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:246–257
Munn II (2003) Aberrant vascular architecture in tumors and its importance in drug-based therapies. Drug Discov Today 8:396–403
Gosh K, Thodeti CK, Dudley AC et al (2008) Tumor-derived endothelial cells exhibit aberrant Rhon-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A 105:11305–11310
Mastria EM, Chen M, McDaniel JR (2015) Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma. J Control Release 118:52–58
Sun L, Deng X, Yang X et al (2014) Co-delivery of doxorubicin and curcumin by polymeric micelles for improving antitumor efficacy on breast carcinoma. RSC Adv 4:46737–46750
Wang J, Ma W, Tu W (2015) Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci 15:1252–1261
Guffy MM, North JA, Burns CP (1984) Effect of cellular fatty acid alteration on adriamycin sensitivity in cultured L1210 murine leukemia cells. Cancer Res 44:1863–1866
Wissing SA, Kayser O, Muller RH (2004) Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56:1257–1272
Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26:57–64
O’Brien MER, Wigler N, Inbar M et al (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 26:440–449
Shuhendler AJ, Prasad P, Zhang RX et al (2014) Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmuno compromised breast tumor model. Mol Pharmaceutics 14:2659–2674
Financial Support
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) for their financial support and fellowships.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 426 kb).
Rights and permissions
About this article
Cite this article
Fernandes, R.S., Silva, J.O., Mussi, S.V. et al. Nanostructured Lipid Carrier Co-loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol Imaging Biol 20, 437–447 (2018). https://doi.org/10.1007/s11307-017-1133-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1133-3