Abstract
Purpose
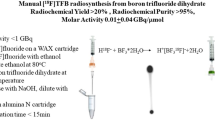
The aim of this study was the automated synthesis of the mitochondrial membrane potential sensor 4-[18F]fluorobenzyl-triphenylphosphonium ([18F]FBnTP) on a commercially available synthesizer in activity yields (AY) that allow for imaging of multiple patients.
Procedures
A three-pot, four-step synthesis was implemented on the ELIXYS FLEX/CHEM radiosynthesizer (Sofie Biosciences) and optimized for radiochemical yield (RCY), radiochemical purity (RCP) as well as chemical purity during several production runs (n = 24). The compound was purified by solid-phase extraction (SPE) with a Sep-Pak Plus Accell CM cartridge, thereby avoiding HPLC purification.
Results
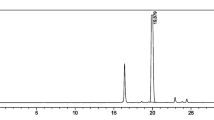
Under optimized conditions, AY of 1.4–2.2 GBq of [18F]FBnTP were obtained from 9.4 to 12.0 GBq [18F]fluoride in 90–92 min (RCY = 28.6 ± 5.1 % with n = 3). Molar activities ranged from 80 to 99 GBq/μmol at the end of synthesis. RCP of final formulations was > 99 % at the end of synthesis and > 95 % after 8 h. With starting activities of 23.2–33.0 GBq, RCY decreased to 16.1 ± 0.4 % (n = 3). The main cause of the decline in RCY when high amounts of [18F]fluoride are used is radiolytic decomposition of [18F]FBnTP during SPE purification.
Conclusions
In initial attempts, the probe was synthesized with RCY < 0.6 % when starting activities up to 44.6 GBq were used. Rapid radiolysis of the intermediate 4-[18F]fluorobenzaldehyde and the final product [18F]FBnTP during purification was identified as the main cause for low yields in high-activity runs. Radiolytic decomposition was hindered by the addition of radical scavengers during synthesis, purification, and formulation, thereby improving AY and RCP. The formulated probe in injectable form was synthesized without the use of HPLC and passed all applicable quality control tests.


Similar content being viewed by others
References
Montgomery MK, Turner N (2015) Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4:R1–R15
Dorn GW, Vega RB, Kelly DP (2015) Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 29:1981–1991
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342:619–630
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11:9–15
Zhang Y, Avalos JL (2017) Traditional and novel tools to probe the mitochondrial metabolism in health and disease. WIREs Syst Biol Med 9(n/a):e1373. https://doi.org/10.1002/wsbm.1373
Ehrenberg B, Montana V, Wei MD et al (1988) Membrane potential can be determined in individual cells from the Nernstian distribution of cationic dyes. Biophys J 53:785–794
Fukuda H, Syrota P, Charbonneau P et al (1986) Use of 11C-triphenylmethylphosphonium for the evaluation of membrane potential in the heart by positron-emission tomography. Eur J Nucl Med 11:478–483
Madar I, Anderson JH, Szabo Z et al (1999) Enhanced uptake of [11C]TPMP in canine brain tumor: a PET study. J Nucl Med 40:1180–1185
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A 97:9226–9233
Ravert HT, Madar I, Dannals RF (2004) Radiosynthesis of 3-[18F]fluoropropyl and 4-[18F]fluorobenzyl triarylphosphonium ions. J Label Compd Radiopharm 47:469–476
Madar I, Huang Y, Ravert H et al (2009) Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med 50:774–780
Madar I, Isoda T, Finley P, Angle J, Wahl R (2011) 18F-fluorobenzyl triphenyl phosphonium: a noninvasive sensor of brown adipose tissue thermogenesis. J Nucl Med 52:808–814
Higuchi T, Fukushima K, Rischpler C et al (2011) Stable delineation of the ischemic area by the PET perfusion tracer 18F-fluorobenzyl triphenyl phosphonium after transient coronary occlusion. J Nucl Med 52:965–969
Madar I, Ravert HT, Du Y et al (2006) Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med 47:1359–1366
Ravert HT, Holt DP, Dannals RF (2014) A microwave radiosynthesis of the 4-[18F]-fluorobenzyltriphenylphosphonium ion. J Label Compd Radiopharm 57:695–698
Zhang Z, Zhang C, Lau J et al (2016) One-step synthesis of 4-[18F]fluorobenzyltriphenylphosphonium cation for imaging with positron emission tomography. J Label Compd Radiopharm 59:467–471
Sanford MS, Scott PJH (2016) Moving metal-mediated 18F-fluorination from concept to clinic. ACS Cent Sci 2:128–130
Lazari M, Collins J, Shen B et al (2014) Fully automated production of diverse 18F-labeled PET tracers on the ELIXYS multireactor radiosynthesizer without hardware modification. J Nucl Med Technol 42:203–210
Claggett SB, Quinn KM, Lazari M et al (2013) Simplified programming and control of automated radiosynthesizers through unit operations. Eur J Nucl Med Mol Imaging Res 3:53
Speranza A, Ortosecco G, Castaldi E et al (2009) Fully automated synthesis procedure of 4-[18F]fluorobenzaldehyde by commercial synthesizer: amino-oxi peptide labelling prosthetic group. Appl Radiat Isot 67:1664–1669
Poethko T, Schottelius M, Thumshirn G et al (2004) Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med 45:892–902
Scott PJH, Hockley BG, Kung HF et al (2009) Studies into radiolytic decomposition of fluorine-18 labeled radiopharmaceuticals for positron emission tomography. Appl Radiat Isot 67:88–94
Schueller MJ, Alexoff DL, Schlyer DJ (2007) Separating long-lived metal ions from 18F during H2 18O recovery. Nucl Instrum Methods Phys Res Sect B 261:795–799
Iwata R, Pascali C, Bogni A et al (2000) A new, convenient method for the preparation of 4-[18F]fluorobenzyl halides. Appl Radiat Isot 52:87–92
Rodnick ME, Brooks AF, Hockley BG et al (2013) A fully-automated one-pot synthesis of [18F]fluoromethylcholine with reduced dimethylaminoethanol contamination via [18F]fluoromethyl tosylate. Appl Radiat Isot 78:26–32
Su D, Cheng Y, Liu M et al (2013) Comparison of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS One 8:e54505
Acknowledgements
We would like to thank Dr. Michael E. Phelps for support and guidance with this study; Dr. Roger Slavik, Krzysztof Bobinski, and Daniel Yeh for providing [18F]fluoride; Dr. Jason Lee, Dr. Tove Olafsen, and Charles Zamilpa for their help with the small animal imaging; and Dr. Michael van Dam, Jeffrey Collins as well as Krzysztof Bobinski for valuable technical input.
Funding
The authors gratefully acknowledge the support from NIH through program, research, and training grants (CA186842, CA208642 and CA086306) and the support from the Department of Energy (DE-SC0012353).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Waldmann, C.M., Gomez, A., Marchis, P. et al. An Automated Multidose Synthesis of the Potentiometric PET Probe 4-[18F]Fluorobenzyl-Triphenylphosphonium ([18F]FBnTP). Mol Imaging Biol 20, 205–212 (2018). https://doi.org/10.1007/s11307-017-1119-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1119-1