Abstract
Purpose
Tumor-associated macrophages (TAMs) are often associated with a poor prognosis in cancer. To gain a better understanding of cellular recruitment and dynamics of TAM biology during cancer progression, we established a novel transgenic mouse model for in vivo imaging of luciferase-expressing macrophages.
Procedures
B6.129P2-Lyz2tm1(cre)Ifo/J mice, which express Cre recombinase under the control of the lysozyme M promoter (LysM) were crossed to Cre-lox Luc reporter mice (RLG), to produce LysM-LG mice whose macrophages express luciferase. Cell-type-specific luciferase expression in these mice was verified by flow cytometry, and via in vivo bioluminescence imaging under conditions where macrophages were either stimulated with lipopolysaccharide or depleted with clodronate liposomes. The distribution of activated macrophages was longitudinally imaged in two immunocompetent LysM-LG mouse models with either B16 melanoma or ID8 ovarian cancer cells.
Results
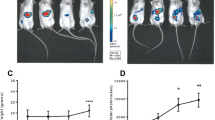
In vivo imaging of LysM-LG mice showed luciferase activity was generated by macrophages. Clodronate liposome-mediated depletion of macrophages lowered overall bioluminescence while lipopolysaccharide injection increased macrophage bioluminescence in both the B16 and ID8 models. Tracking macrophages weekly in tumor-bearing animals after intraperitoneal (i.p.) or intraovarian (i.o.) injection resulted in distinct, dynamic patterns of macrophage activity. Animals with metastatic ovarian cancer after i.p. injection exhibited significantly higher peritoneal macrophage activity compared to animals after i.o. injection.
Conclusion
The LysM-LG model allows tracking of macrophage recruitment and activation during disease initiation and progression in a noninvasive manner. This model provides a tool to visualize and monitor the benefit of pharmacological interventions targeting macrophages in preclinical models.





Similar content being viewed by others
References
Pollard JW (2009) Trophic macrophages in development and disease. Nat Rev Immunol 9:259–270
De Palma M, Lewis CE (2013) Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23:277–286
Cook J, Hagemann T (2013) Tumour-associated macrophages and cancer. Curr Op Pharm 13:595–601
Takaishi K, Komohara Y, Tashiro H et al (2010) Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci 101:2128–2136
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182:4499–4506
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Solinas G, Marchesi F, Garlanda C et al (2010) Inflammation-mediated promotion of invasion and metastasis. Cancer Met Rev 29:243–248
Mantovani A, Sozzani S, Locati M et al (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Zhang M, He Y, Sun X et al (2014) A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 7:19
Hagemann T, Wilson J, Burke F et al (2006) Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol 176:5023–5032
Ruffell B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol 33:119–126
Daldrup-Link H, Coussens LM (2012) MR imaging of tumor-associated macrophages. Oncoimmunology 1:507–509
Daldrup-Link HE, Golovko D, Ruffell B et al (2011) MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res 17:5695–5704
Clausen BE, Burkhardt C, Reith W et al (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277
Cross M, Mangelsdorf I, Wedel A, Renkawitz R (1988) Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A 85:6232–6236
Kanada M, Bachmann MH, Hardy JW et al (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 112:E1433–E1442
Gonzalez-Gonzalez E, Ra H, Hickerson RP et al (2009) siRNA silencing of keratinocyte-specific GFP expression in a transgenic mouse skin model. Gene Ther 16:963–972
Contag CH, Jenkins D, Contag PR, Negrin RS (2000) Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2:41–52
Edinger M, Sweeney TJ, Tucker AA et al (1999) Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia 1:303–310
Chow A, Brown BD, Merad M (2011) Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11:788–798
Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Moughon DL, He H, Schokrpur S et al (2015) Macrophage blockade using CSF1R inhibitors reverses the vascular leakage underlying malignant ascites in late-stage epithelial ovarian cancer. Cancer Res 75:4742–4752
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61
Burnett SH, Kershen EJ, Zhang J et al (2004) Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol 75:612–623
Evrard M, Chong SZ, Devi S et al (2015) Visualization of bone marrow monocyte mobilization using Cx3cr1gfp/+Flt3L−/− reporter mouse by multiphoton intravital microscopy. J Leukoc Biol 97:611–619
Geissmann F, Manz MG, Jung S et al (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8:533–544
Pyonteck SM, Akkari L, Schuhmacher AJ et al (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Med 19:1264–1272
Schreiber HA, Loschko J, Karssemeijer RA et al (2013) Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med 210:2025–2039
Long KB, Beatty GL (2013) Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology 2:e26860
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795
Guiducci C, Vicari AP, Sangaletti S et al (2005) Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 65:3437–3446
Ribas A, Wolchok JD (2013) Combining cancer immunotherapy and targeted therapy. Curr Op Immunol 25:291–296
Garris C, Pittet MJ (2013) Therapeutically reeducating macrophages to treat GBM. Nature Med 19:1207–1208
Chitu V, Stanley ER (2006) Colony-stimulating factor-1 in immunity and inflammation. Curr Op Immunol 18:39–48
Sasmono RT, Oceandy D, Pollard JW et al (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101:1155–1163
Hume DA (2011) Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J Leukoc Biol 89:525–538
Fantin A, Vieira JM, Gestri G et al (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116:829–840
Clarke S, Greaves DR, Chung LP et al (1996) The human lysozyme promoter directs reporter gene expression to activated myelomonocytic cells in transgenic mice. Proc Natl Acad Sci U S A 93:1434–1438
Keshav S, Chung P, Milon G, Gordon S (1991) Lysozyme is an inducible marker of macrophage activation in murine tissues as demonstrated by in situ hybridization. J Exp Med 174:1049–1058
Fenrich KK, Weber P, Rougon G, Debarbieux F (2013) Long- and short-term intravital imaging reveals differential spatiotemporal recruitment and function of myelomonocytic cells after spinal cord injury. J Physiol 591:4895–4902
Acknowledgements
This work was supported by the Mary Lake Polan Gynecologic Oncology Endowment for Research (O. D.), the Vivian Scott Fellowship in Gynecologic Oncology (O. D.), the Dean Pizzo Stanford Cancer Center Research Award (O. D.), the Child Health Research Institute at Stanford (C. C.), and a generous gift from the Chambers Family Foundation for Excellence in Pediatric Research (C.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 347 kb)
Rights and permissions
About this article
Cite this article
He, H., Chiu, A.C., Kanada, M. et al. Imaging of Tumor-Associated Macrophages in a Transgenic Mouse Model of Orthotopic Ovarian Cancer. Mol Imaging Biol 19, 694–702 (2017). https://doi.org/10.1007/s11307-017-1061-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1061-2