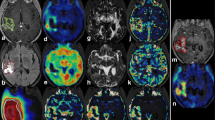
Abstract
Purpose
Diffusion magnetic resonance imaging (MRI) and 6-[18F]fluoro-l-dopa ([18F]FDOPA) positron emission tomography (PET) are used to interrogate malignant tumor microenvironment. It remains unclear whether there is a relationship between [18F]FDOPA uptake, diffusion MRI estimates of apparent diffusion coefficient (ADC), and mitotic activity in the context of recurrent malignant gliomas, where the tumor may be confounded by the effects of therapy. The purpose of the current study is to determine whether there is a correlation between these imaging techniques and mitotic activity in malignant gliomas.
Procedures
We retrospectively examined 29 patients with recurrent malignant gliomas who underwent structural MRI, diffusion MRI, and [18F]FDOPA PET prior to surgical resection. Qualitative associations were noted, and quantitative voxel-wise and median measurement correlations between [18F]FDOPA PET, ADC, and mitotic index were performed.
Results
Areas of high [18F]FDOPA uptake exhibited low ADC and areas of hyperintensity T2/fluid-attenuated inversion recovery (FLAIR) with low [18F]FDOPA uptake exhibited high ADC. There was a significant inverse voxel-wise correlation between [18F]FDOPA and ADC for all patients. Median [18F]FDOPA uptake and median ADC also showed a significant inverse correlation. Median [18F]FDOPA uptake was positively correlated, and median ADC was inversely correlated with mitotic index from resected tumor tissue.
Conclusions
A significant association may exist between [18F]FDOPA uptake, diffusion MRI, and mitotic activity in recurrent malignant gliomas.



Similar content being viewed by others
References
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Bode MK, Ruohonen J, Nieminen MT, Pyhtinen J (2006) Potential of diffusion imaging in brain tumors: a review. Acta Radiol 47:585–594
Ellingson BM, Malkin MG, Rand SD et al (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31:538–548
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Lyng H, Haraldseth O, Rofstad EK (2000) Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 43:828–836
Chenevert TL, Stegman LD, Taylor JM et al (2000) Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 92:2029–2036
Guo AC, Cummings TJ, Dash RC, Provenzale JM (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224:177–183
Hayashida Y, Hirai T, Morishita S et al (2006) Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol 27:1419–1425
Kinoshita M, Hashimoto N, Goto T et al (2008) Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 43:29–35
Chenevert TL, McKeever PE, Ross BD (1997) Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res Off J Am Assoc Cancer Res 3:1457–1466
Ross BD, Moffat BA, Lawrence TS et al (2003) Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther 2:581–587
Moffat BA, Hall DE, Stojanovska J et al (2004) Diffusion imaging for evaluation of tumor therapies in preclinical animal models. Magma 17:249–259
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Asao C, Korogi Y, Kitajima M et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26:1455–1460
Sundgren PC, Fan X, Weybright P et al (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24:1131–1142
Weber WA, Wester HJ, Grosu AL et al (2000) O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 27:542–549
Becherer A, Karanikas G, Szabo M et al (2003) Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging 30:1561–1567
Grosu AL, Astner ST, Riedel E et al (2011) An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys 81:1049–1058
Isselbacher KJ (1972) Sugar and amino acid transport by cells in culture–differences between normal and malignant cells. N Engl J Med 286:929–933
Busch H, Davis JR, Honig GR et al (1959) The uptake of a variety of amino acids into nuclear proteins of tumors and other tissues. Cancer Res 19:1030–1039
Kato T, Shinoda J, Oka N et al (2008) Analysis of 11C-methionine uptake in low-grade gliomas and correlation with proliferative activity. AJNR Am J Neuroradiol 29:1867–1871
Sato N, Suzuki M, Kuwata N et al (1999) Evaluation of the malignancy of glioma using 11C-methionine positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg Rev 22:210–214
Chiang PK, Cantoni GL (1977) Activation of methionine for transmethylation. Purification of the S-adenosylmethionine synthetase of bakers’ yeast and its separation into two forms. J Biol Chem 252:4506–4513
Meyer GJ, Schober O, Hundeshagen H (1985) Uptake of 11C-L- and D-methionine in brain tumors. Eur J Nucl Med 10:373–376
Souba WW (1993) Glutamine and cancer. Ann Surg 218:715–728
Yamada Y, Uchida Y, Tatsumi K et al (1998) Fluorine-18-fluorodeoxyglucose and carbon-11-methionine evaluation of lymphadenopathy in sarcoidosis. J Nucl Med 39:1160–1166
Floeth FW, Pauleit D, Sabel M et al (2006) 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med 47:776–782
Fueger BJ, Czernin J, Cloughesy T et al (2010) Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nucl Med 51:1532–1538
Kinahan PE, Townsend DW, Beyer T, Sashin D (1998) Attenuation correction for a combined 3D PET/CT scanner. Med Phys 25:2046–2053
Nuyts J, Michel C, Dupont P (2001) Maximum-likelihood expectation-maximization reconstruction of sinograms with arbitrary noise distribution using NEC-transformations. IEEE Trans Med Imaging 20:365–375
Chen W, Silverman DH, Delaloye S et al (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
Johannessen AL, Torp SH (2006) The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res POR 12:143–147
Prayson RA (2005) The utility of MIB-1/Ki-67 immunostaining in the evaluation of central nervous system neoplasms. Adv Anat Pathol 12:144–148
Hatakeyama T, Kawai N, Nishiyama Y et al (2008) 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging 35:2009–2017
Huang MC, Shih YH, Chen MH et al (2005) Malignancy of intracerebral lesions evaluated with 11C-methionine-PET. J Clin Neurosci Off J Neurosurg Soc Australas 12:775–780
Kim S, Chung JK, Im SH et al (2005) 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 32:52–59
Yuan W, Holland SK, Jones BV et al (2008) Characterization of abnormal diffusion properties of supratentorial brain tumors: a preliminary diffusion tensor imaging study. J Neurosurg Pediatr 1:263–269
Gauvain KM, McKinstry RC, Mukherjee P et al (2001) Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am J Roentgenol 177:449–454
Conflict of Interest
The authors have no conflict of interest concerning the subject matter in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research Support
NIH/NCI R21CA167354 (BME), UCLA Institute for Molecular Medicine Seed Grant (BME), UCLA Jonsson Comprehensive Cancer Center Seed Grant (BME), UCLA Radiology Exploratory Research Grant (BME), University of California Cancer Research Coordinating Committee Grant (BME), ACRIN Young Investigator Initiative Grant (BME), National Brain Tumor Society Research Grant (BME), Siemens Healthcare Research Grant (BME), Art of the Brain (TFC), Ziering Family Foundation in memory of Sigi Ziering (TFC), Singleton Family Foundation (TFC), and Clarence Klein Fund for Neuro-Oncology (TFC).
Rights and permissions
About this article
Cite this article
Karavaeva, E., Harris, R.J., Leu, K. et al. Relationship Between [18F]FDOPA PET Uptake, Apparent Diffusion Coefficient (ADC), and Proliferation Rate in Recurrent Malignant Gliomas. Mol Imaging Biol 17, 434–442 (2015). https://doi.org/10.1007/s11307-014-0807-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0807-3